Pneumonia
This page was first published in November 2019, and we updated the text in February 2024.
Pneumonia is one of the most common causes of death worldwide. It is a condition of the inflammation of the lungs, specifically in the alveoli, which are millions of tiny air sacs that help us take in oxygen.
In pneumonia, these alveoli become filled with pus and fluid, which makes breathing painful and reduces our ability to take in oxygen from the air we breathe and exhale carbon dioxide. Pneumonia is one of the leading causes of death worldwide.
It can develop from a range of different infections, which are caused by different pathogens, including viruses, bacteria, and fungi. This includes, for example, Streptococcus pneumoniae, Haemophilus influenzae, Staphylococcus aureus, influenza (flu), respiratory syncytial virus (RSV), and more.
These pathogens are contagious and can spread when a person coughs or sneezes.
On this topic page, we look at who is suffering from pneumonia and why — and what can we do to reduce the number of people dying from this disease, with interventions such as vaccination, oxygen therapy, reducing air pollution, and more.
See all interactive charts on pneumonia ↓
Related topics
Other research and writing on pneumonia on Our World in Data:
Burden of pneumonia
Number of people dying from pneumonia by age
The chart here shows the global number of deaths from pneumonia1 by age group.
The number of children dying from pneumonia has decreased substantially over the past three decades. In 1990, more than two million children died from pneumonia every year. By 2019, this number had fallen by almost two-thirds.
Reductions in the major risk factors such as childhood wasting, air pollution, and poor sanitation, as well as falling global poverty and better availability of health technology, such as pneumococcal vaccines and antibiotics, have all contributed to this decline.
The number of deaths among those aged 70 and older increased — from around 600,000 in 1990 to over 1 million in 2019. This is largely because of a growing and aging population. The death rate from pneumonia in this age group fell slightly.

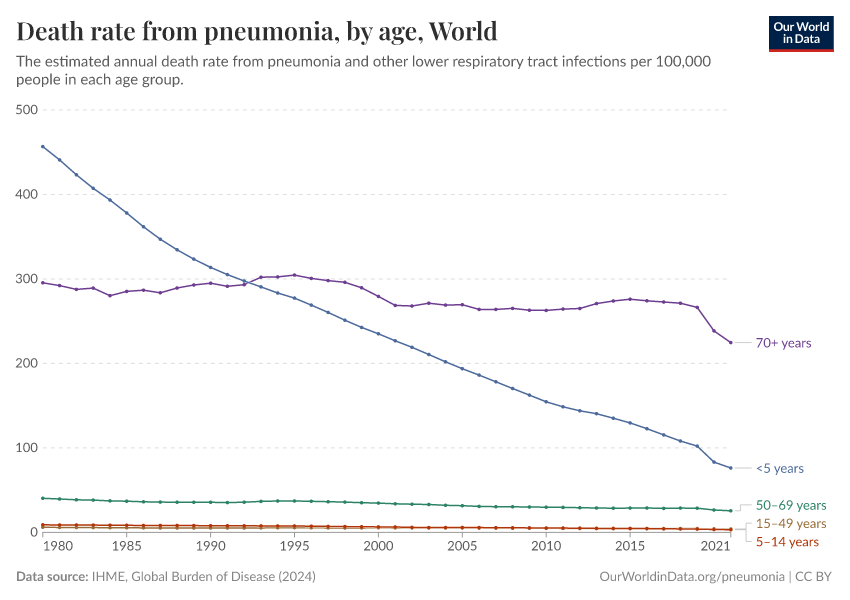
Pneumonia mortality rates by age
The chart shows the annual death rate from pneumonia per 100,000 people in different age groups.
Among children under five, the death rate from pneumonia has declined substantially since 1990.
In 2019, the highest pneumonia death rates were among people aged 70 and older.
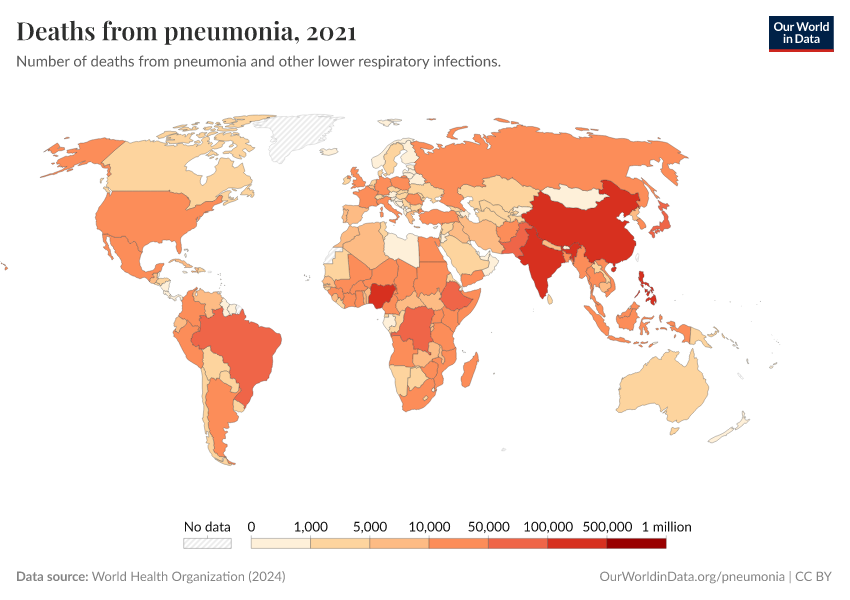
Where do people die from pneumonia?
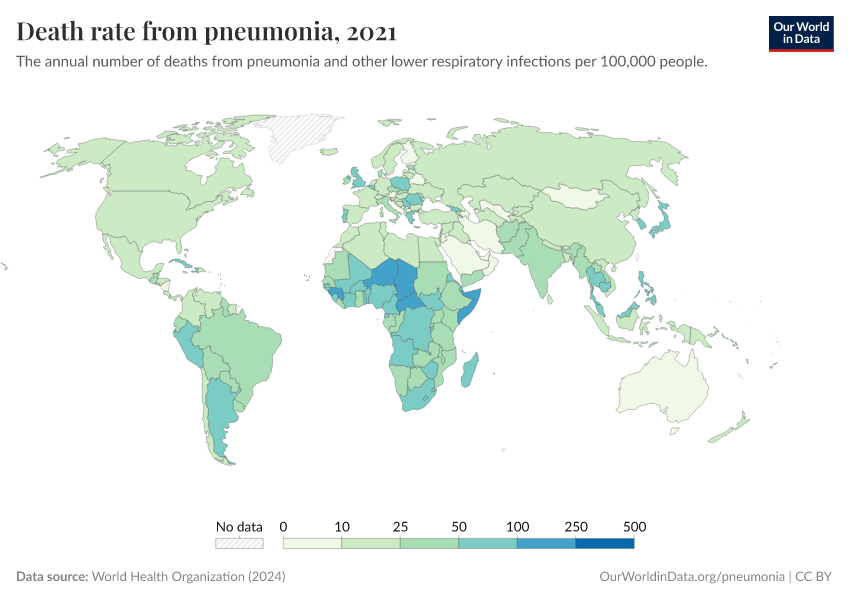
Pneumonia death rates — all ages
The map below shows the annual death rate from pneumonia, per 100,000 people in the population.
The death rates are age-standardized, which helps make comparisons between populations of different age structures.
As you can see, the difference between death rates in different world regions is very large. The death rate from pneumonia is highest in sub-Saharan Africa and South East Asia, and much lower in Europe and North America.

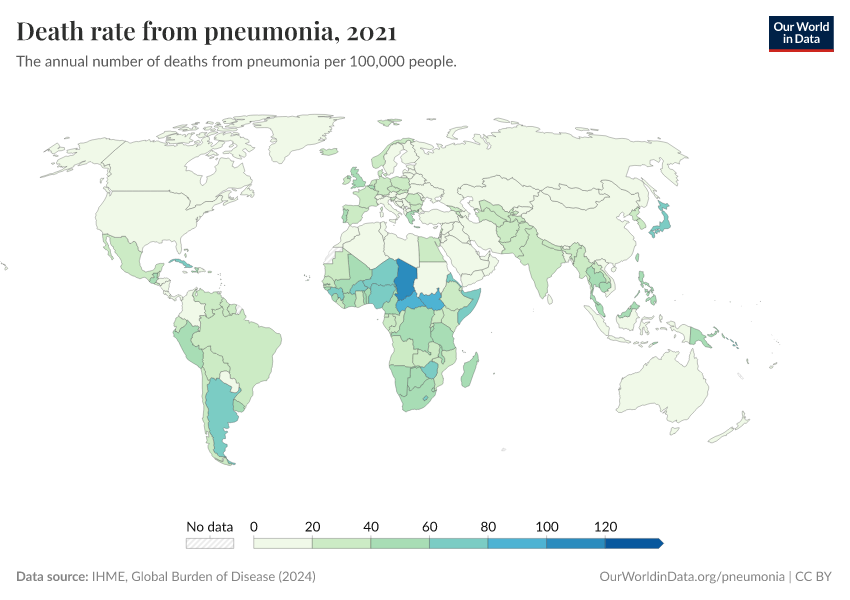
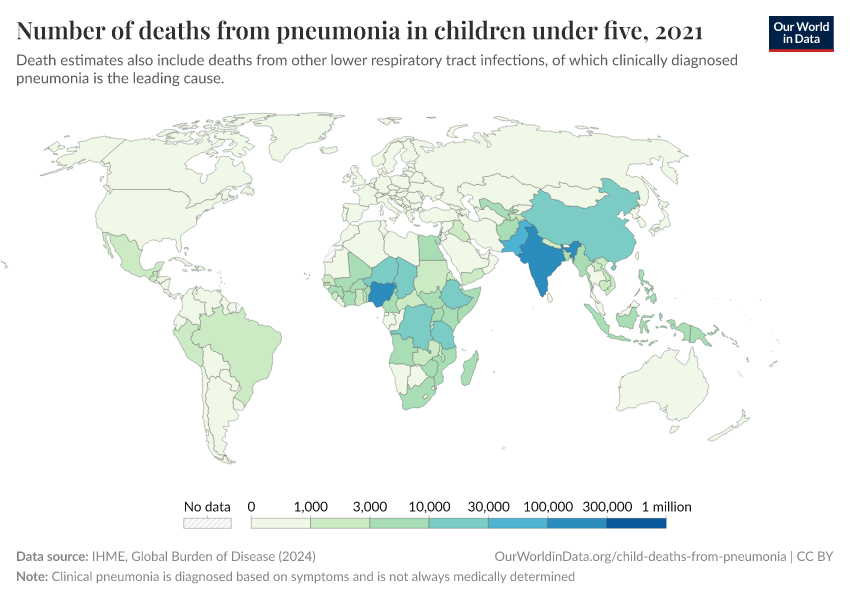
Where do children die from pneumonia
The map below shows the annual death rate from pneumonia among children under five specifically.
It shows that children are most likely to die from pneumonia across Sub-Saharan Africa and South Asia.

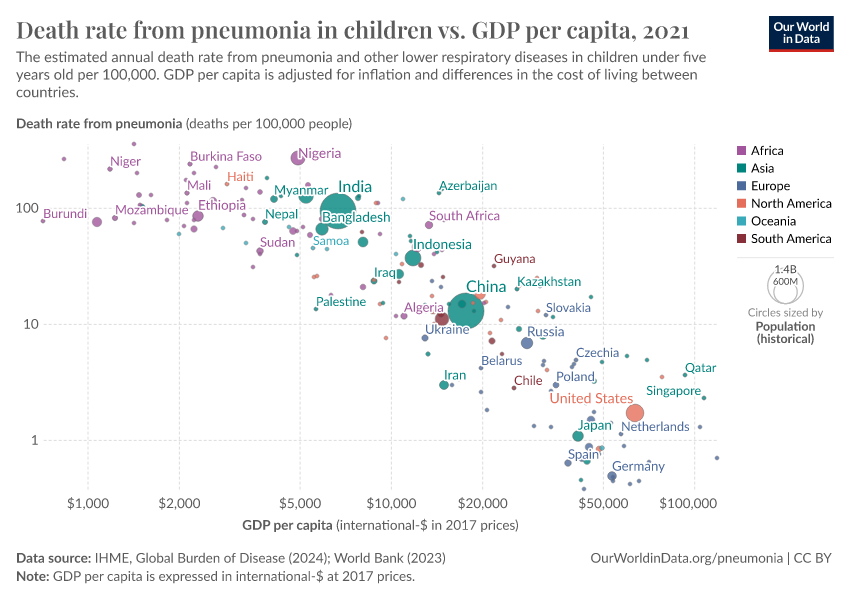
For this reason, Kevin Watkins and Devi Sridhar called pneumonia “the ultimate disease of poverty” in a 2018 comment in the journal The Lancet.2
There is a very strong correlation between a country’s income and the child mortality rate from pneumonia as the scatter plot shows.
The disease is most common in poor places where healthcare infrastructure is lacking and people are least able to afford the treatment.3
Read more in our article:

How is pneumonia defined in global estimates?
Ideally, people with pneumonia would receive a specific diagnosis for the condition — to identify that the alveoli in their lungs are affected — and also be diagnosed with the specific infectious cause of pneumonia.
But, in many cases, this doesn’t occur, especially when people lack healthcare access and advanced diagnostic capacity.
This is why academic studies use the terms “clinical pneumonia” or “WHO pneumonia”, which refers to cases of pneumonia based on symptoms alone — most importantly, fast breathing and coughing. This definition inevitably means that other diseases with similar symptoms may be counted as cases of pneumonia.
As a consequence, the terms “pneumonia” and “lower respiratory infections” are often used interchangeably.
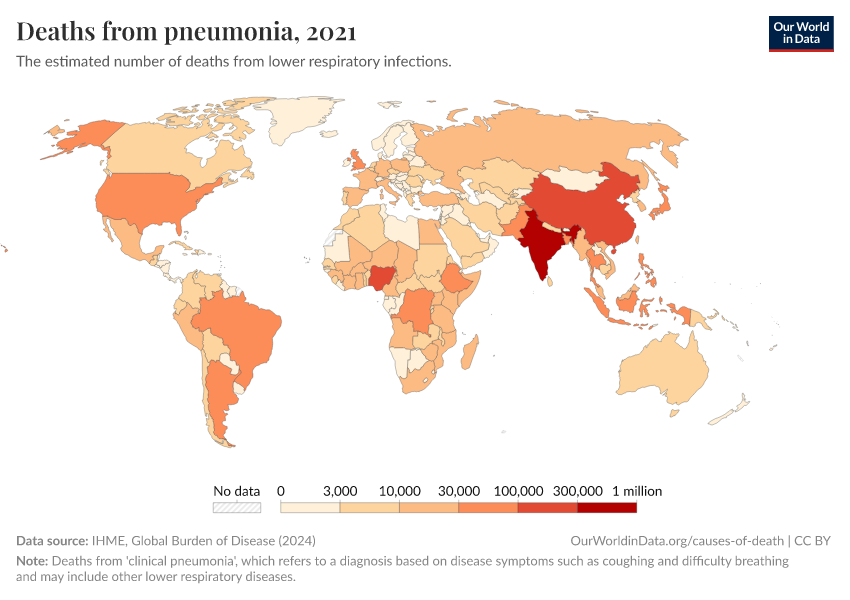
The Institute for Health Metrics and Evaluation (IHME), for example, provides data on deaths from lower respiratory infections, including pneumonia caused by a range of different pathogens as well as bronchiolitis (a lower respiratory tract infection that mostly affects very young children) in the same category.4 5
While cases of bronchiolitis are quite common, they are milder, and estimates of the number of deaths from lower respiratory infections generally refer to deaths from infections that cause pneumonia and other lower respiratory infections.
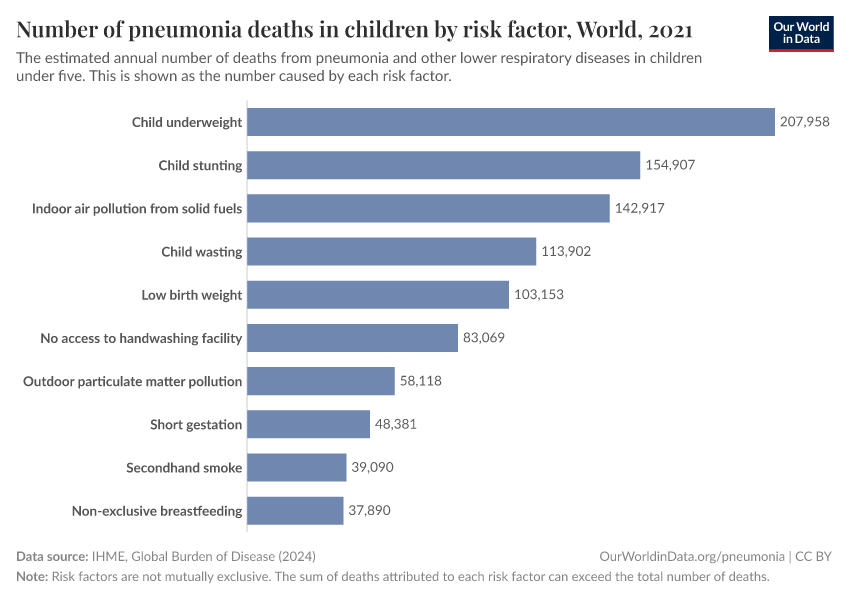
What are the biggest risks for developing pneumonia?
Why are children dying from pneumonia?
Despite progress against pneumonia, hundreds of thousands of children still die from the condition each year.
To understand how we can reduce the number of children dying from pneumonia we need to understand both prevention and treatment.
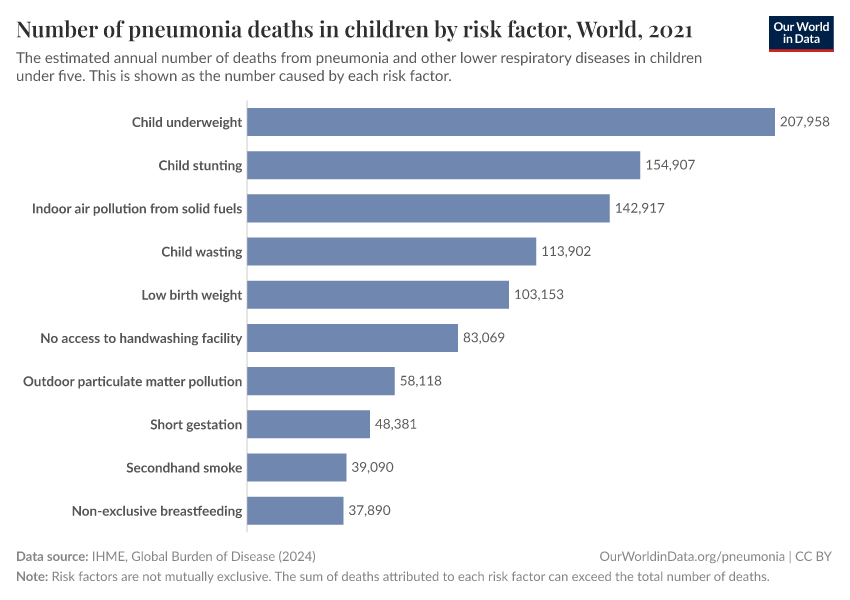
The chart shows the estimated number of pneumonia deaths caused by different risk factors, in children.
Undernutrition is the major contributor to pneumonia mortality
The chart shows that childhood undernutrition — especially "child wasting" (children who have a weight too low for their height) — is a major risk factor for pneumonia in children.6
Without sufficient energy intake, the body cannot cope with the increased energy demands required to fight off infections.
A literature review of pneumonia in malnourished children by Mohammod Jobayer Chisti and colleagues found that undernourished children are between two to four times more likely to be admitted to hospital due to pneumonia, and up to 15 times more likely to die from it.7
Air pollution and second-hand smoke increase the risk of getting pneumonia
Air pollution is also a major risk factor for pneumonia, as you can see in the chart.
This includes both indoor air pollution and outdoor air pollution.
Studies have shown that high indoor air pollution in households can double the chances that children develop pneumonia and make recovery more difficult.8
One reason is that small polluting particles impair the immune system’s ability to fight and clear the infection.
Laura Jones et al. (2011) reviewed studies on the impact of secondhand smoke on children, and concluded that children who live in households with smoking parents are more likely to acquire pneumonia as well as other respiratory illnesses.9
The data shows estimates of the global number of pneumonia deaths attributed to secondhand smoke.
The risk of pneumonia is higher for children with HIV
Other infectious diseases, such as measles and HIV, also increase the risk of pneumonia in children.
When children who are infected with HIV develop AIDS — which weakens their immune system — their chances of dying from pneumonia increase substantially.
A study by Evropi Theodoratou et al., published in Lancet Infectious Diseases, found that children with HIV have a seven times greater risk of dying from pneumonia than those without it.10
Read more on our page on HIV/AIDS:
Overcrowding facilitates pneumonia transmission
Pathogens that cause pneumonia tend to be more easily transmitted between people who are living together — through close contact, respiratory droplets, or airborne particles.
Therefore, overcrowding — too many people living in one space — also increases the risks of pneumonia.
This is yet another reason why pneumonia is a disease of poverty, children in low and middle-income countries are more likely to live in overcrowded households.11
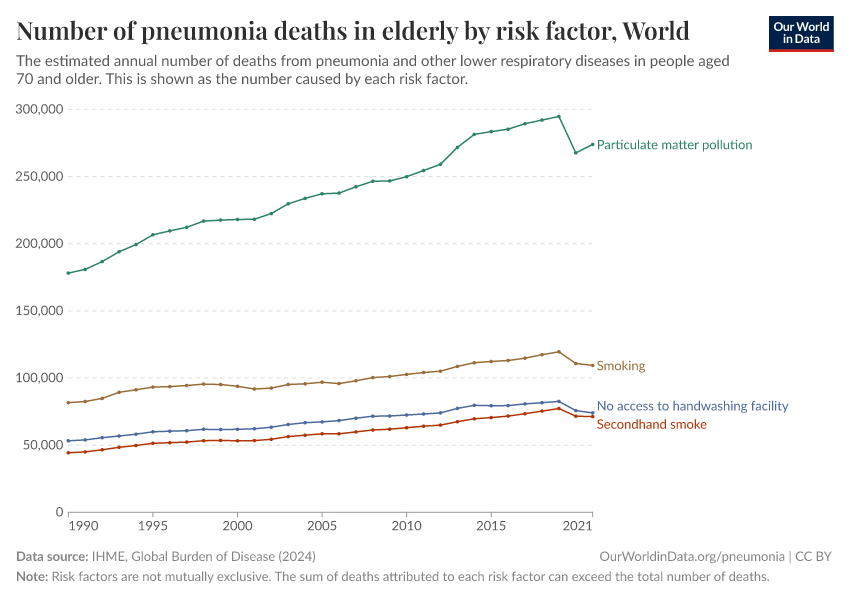
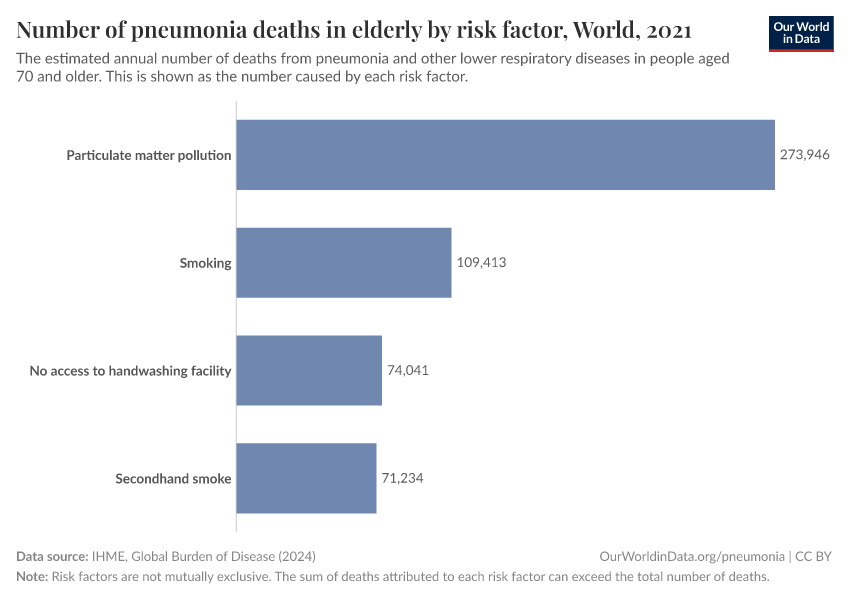
Pneumonia risk factors for people aged 70 and older
The risk factors for developing pneumonia in people aged 70 and older are similar to the risk factors that lead to pneumonia in children.
Outdoor air pollution — small particulate matter air pollution — is a major risk factor for dying from pneumonia, as you can see in the chart.
In addition, smoking and exposure to secondhand smoke are also important risk factors.
How can we reduce the number of people dying from pneumonia?
Oxygen therapy
One of the main risks of pneumonia is breathing difficulties, and people become unable to take in oxygen properly.
When pneumonia develops, the alveoli in the lungs get filled with pus and fluid, which prevents oxygen from being transferred to the blood.
Consequently, a condition known as hypoxemia — which means a lack of oxygen in the blood — can develop.
A study by Marzia Lazzerini et al. finds that children with pneumonia who develop hypoxemia have a five times higher risk of death.12
To treat this condition, oxygen therapy — supplying oxygen-enriched air to the patient — can be very important.13
Since 2017, the WHO has included oxygen in its “List of Essential Medicines”.14
Pneumococcal vaccines
Since pneumonia is caused by different infections, a range of vaccines can be used to protect against it, including pneumococcal vaccines, Hib vaccines, influenza vaccines, respiratory syncytial virus (RSV) vaccines, and more.
Pneumococcal vaccines protect against pneumonia from the Streptococcus pneumoniae bacteria, which is one of the major causes of pneumonia in children under 5.15
There are several versions of pneumococcal conjugate vaccine (PCV), which target different serotypes of S. pneumoniae.
The PCV vaccine is given to children younger than 24 months. According to a study by Cheryl Cohen et al. (2017), PVC13 — a common version of the PCV vaccine — reduces the risk of invasive pneumococcal infections by 85%, for the strains included in the vaccine formulation.16
Despite this efficacy, the coverage of pneumococcal vaccines still lags behind other vaccines.
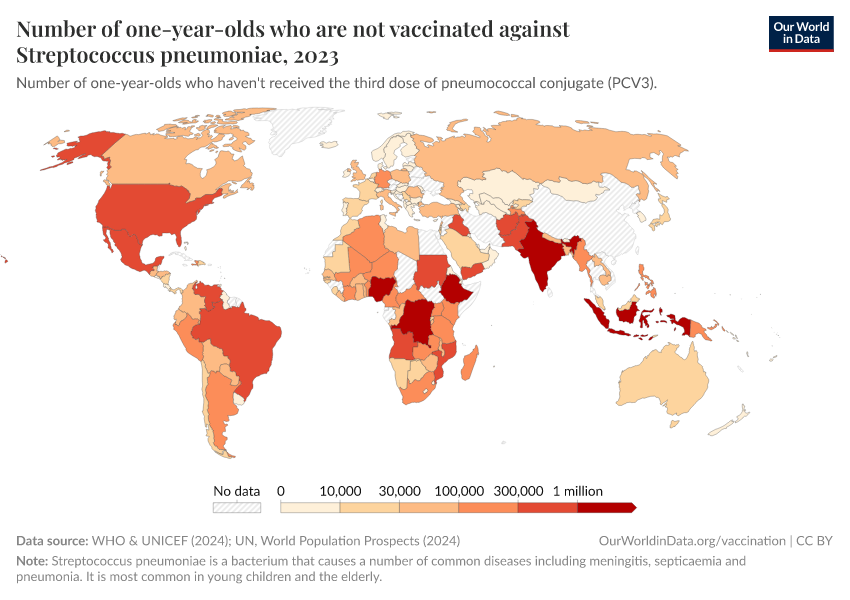
Coverage of pneumococcal vaccination
Since the World Health Organisation (WHO) started recommending including pneumococcal vaccines in national immunisation programmes for children in 2007, there has been a progressive increase in the number of countries using the vaccine, which you can see in the map, by pressing the "Play timelapse" button.

But the coverage of pneumococcal vaccines is still low in many countries, as you can see in the map.
In 2021, around half of one-year-olds worldwide received the third dose of pneumococcal vaccines.
This means that millions of children who could be protected against pneumonia — one of the leading causes of death — were still not vaccinated against it.17

How do pneumococcal vaccines work?
Streptococcus pneumonia, often simply referred to as pneumococcus, is a bacterium that is often found in the upper respiratory tract of healthy people.
Generally, the bacterium is harmless or causes milder illnesses such as bronchitis, sinusitis, and ear infections.
Pneumococcal vaccines are effective against these milder illnesses as well, but importantly also protects from what is called “pneumococcal invasive disease”, which is more severe. This severe form of the disease develops when the bacteria moves from the upper respiratory tract to other parts of the body that such as the blood, cerebrospinal fluid or pleural cavity (fluid-filled space surrounding the lungs) — which are usually free of pathogens.18
By invading these parts of the body, the bacteria leads to life-threatening diseases such as sepsis, meningitis and severe pneumonia.
The chart below shows the number of deaths in children from the different diseases that the bacteria can cause.
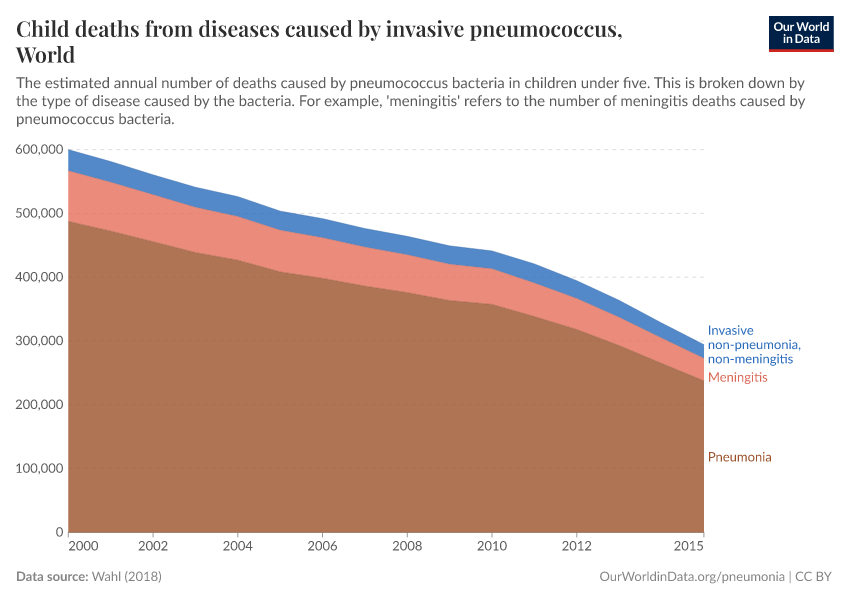
There are two types of pneumococcal vaccines available: conjugated polysaccharide pneumococcal vaccine (PCV) and non-conjugated polysaccharide pneumococcal vaccine (PPSV).
Both types stimulate an immune response against multiple serotypes of the S. pneumoniae bacteria. There are many serotypes of the bacteria — which are defined by the different immune responses to the sugars found on the bacterial surface19 — but only a limited subset of them are responsible for most cases of invasive pneumococcal disease currently.20
How effective are pneumococcal vaccines?
Pneumococcal vaccines are highly effective at reducing the risk of severe pneumonia.
In clinical trials, the PCV vaccines have reduced the risk of invasive pneumococcal disease by 80%, among the serotypes included in the vaccine formulation. In addition, vaccinated children are 27% less likely to be diagnosed with pneumonia overall and 11% less likely to die from it.21
As of 2023, it’s recommended that young children — under the age of two — receive the PCV vaccines, because the PPSV vaccines are not effective at such a young age.22
How many child deaths could be averted by pneumococcal vaccines?
Several studies have attempted to estimate how many lives PCV vaccination has saved and could save.
A study by Brian Wahl et al. (2018) that the number of childhood deaths caused by pneumococcus fell from 600,000 to 294,000 across 120 countries between 2000 and 2015, which is a decline of 54%.
Most of this decline was attributed to the PCV vaccines: over this period, it’s estimated these vaccines saved the lives of 250,000 children. The majority of these deaths would have been caused by pneumonia, but the vaccine also prevented deaths from pneumococcal meningitis and other diseases.23
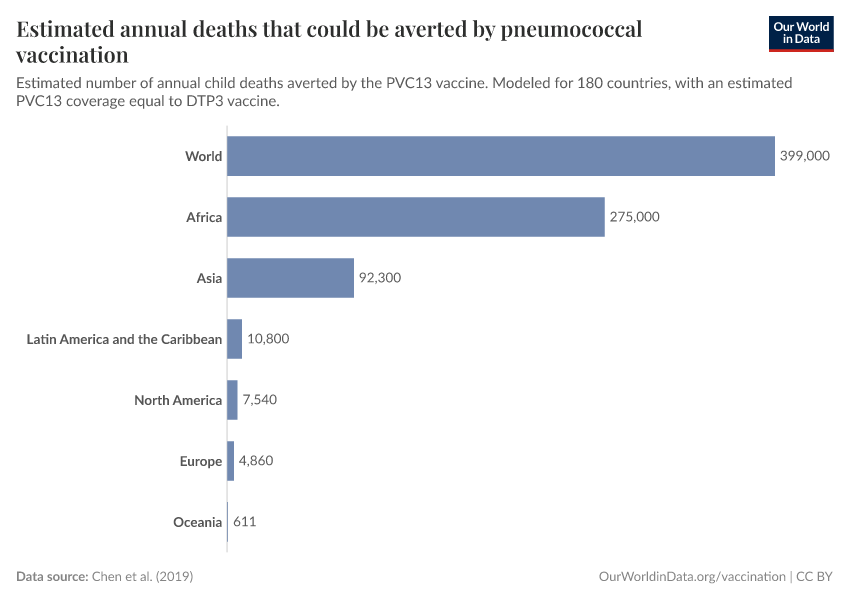
The chart below shows how many more lives the pneumococcal vaccine could save.
It is based on a study by Cynthia Chen et al. (2019), which estimated the number of lives that would be saved if vaccination rates for PCV3 matched vaccination rates of DTP3, which is a vaccine against diphtheria, tetanus, and pertussis.
With this approach, they estimate that, in total, around 400,000 children under 5 could be saved annually.24 In addition, they estimated that around 54.6 million cases of pneumonia could be averted annually.
It’s important to note that these numbers estimate the impact of the PCV vaccination relative to a world without that vaccine. Since the vaccine is already used, some of these lives are already being saved by PCV vaccination.
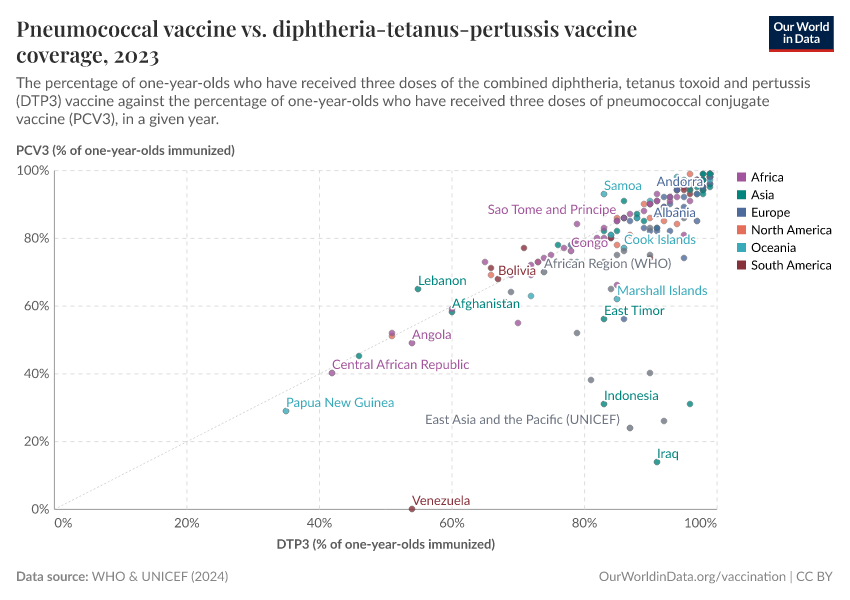
However, in many countries, PCV vaccination rates still fall far below the DTP3 rates, as you can see below. This makes it clear that we still haven’t used the pneumococcal vaccine to its full potential.
What can we do to improve the coverage and effectiveness of pneumococcal vaccines?
To make full potential of pneumococcal vaccines, it’s important to have an increased introduction and coverage of the vaccines in countries around the world.
The cost of PCV vaccines ranges widely: from $3.05 per dose in countries supported by Gavi, the vaccine alliance, which subsidizes the cost of vaccination in low-income countries,25 to $169 in high-income countries, such as the United States.26
For low-middle-income countries who are transitioning away from GAVI support, the increasing future costs of vaccination could place a strain on national healthcare budgets.27
But, because of the high impact of pneumonia, PCV vaccines are considered to be very cost-effective, with an estimated return of investment in low- and middle-income countries of around 3.28
Another way to make full use of the potential of pneumococcal vaccines is by collecting data on which serotypes of S. pneumoniae are common in countries, and adapting vaccines to target these serotypes.
As mentioned previously, PCV vaccines include a limited number of pneumococcal serotypes. The distribution of pneumococcal serotypes is known to vary between countries, and PCV vaccines include the ones that are most common globally.
But the serotypes that are most common in a particular country may affect the potential for a particular vaccine’s impact.
However, not all countries collect data on the serotypes common in countries, which reduces the efficacy of pneumococcal vaccination.29
Since the PCV vaccine was introduced, cases of invasive pneumococcal disease have reduced substantially. But now, the remaining cases tend to be from the less common serotypes, which the vaccine doesn’t protect against.30
This highlights that it’s important to continually collect data on the serotypes affecting a country, and adapt vaccines against a wider range of serotypes. Some fo these vaccines are already in development.31
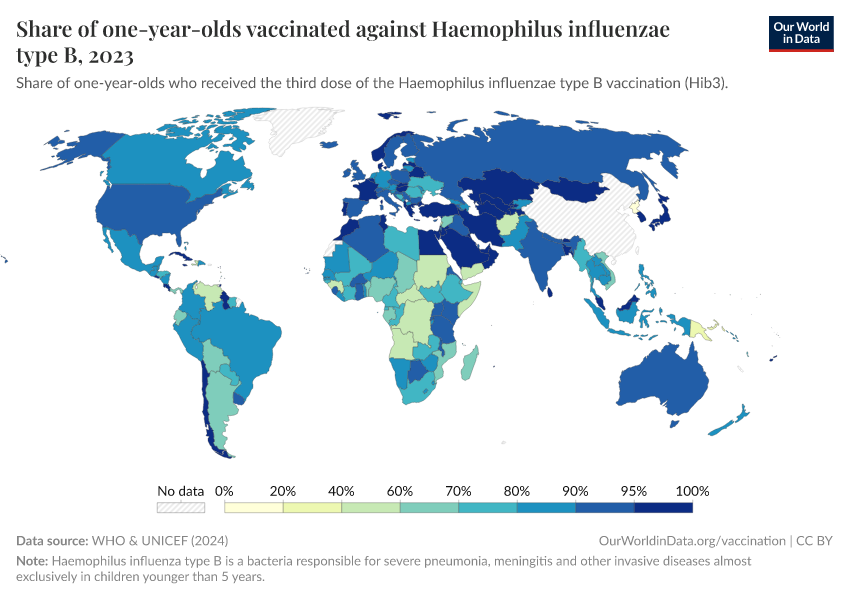
Haemophilus influenzae type b vaccines
Another vaccine widely used to protect children against pneumonia is the Hib vaccine, which protects children against Haemophilus influenzae type b, a major cause of meningitis in children.
Researchers estimate that the Hib vaccine reduces the risk of Hib-related pneumonia by around 70% and the risk of meningitis by around 84%, in children.32
The chart below shows the share of children vaccinated against Haemophilus influenzae type b.
Aside from oxygen therapy and vaccination, other interventions can also help protect children against pneumonia.
Reducing air pollution
There has been significant progress in reducing air pollution levels in recent decades, particularly indoor air pollution.
Death rates from indoor air pollution have fallen as a result of improved access to cleaner fuels for heating and cooking.
But there is still much progress to be made, especially in Sub-Saharan Africa, where most households lack access to clean fuels for cooking.
And, although progress has been made against indoor air pollution, high outdoor pollution remains a problem across many countries.
Reducing air pollution levels would have also many other benefits: it would also reduce the incidence of other diseases such as asthma in children, for example.33
Read more on our page on air pollution:

Access to healthcare and treatment
A child with a suspected case of pneumonia — who has difficulty breathing and has been coughing — should be taken to a healthcare provider so that proper immediate treatment can be provided.
Delays in seeking treatment can increase the risk of a child dying.34
But, as the map shows, seeking healthcare is still not as common as it should be. Globally, less than two-thirds of children with symptoms of pneumonia were taken to a healthcare provider in 2016. This figure is even lower in places where healthcare is most needed — just 47% in Sub-Saharan Africa.35
As the map shows, the share of children with symptoms of pneumonia that are taken to a health provider is still low in many countries.

Access to antibiotic treatment
Given that most cases of pneumonia are caused by bacteria, antibiotics are the general course of treatment.
Due to the lack of resources, in places where pneumonia cases are most common, a quick diagnosis of the cause of the disease is not always possible.
Because of the risk of death from untreated pneumonia, the World Health Organisation (WHO) recommends treatment with antibiotics – depending on the person’s symptoms and their severity — before the cause of disease is known.
Amoxicillin, ampicillin, and gentamicin are the most commonly-used antibiotics to treat pneumonia.36 Antibiotics are a relatively cheap and effective treatment. 37 38
Promoting breastfeeding
Encouraging mothers to breastfeed during the first 6 months of a child’s life can have a positive impact on reducing child undernutrition, and help protect them from some infectious pathogens, such as those that can lead to pneumonia.
According to a study by Laura Lamberti et al. (2013), children in developing countries who are not breastfed in the first five months of their lives have a much higher risk of dying from pneumonia than those who exclusively received their mother’s milk.39
As the map shows, the number of infants who are exclusively breastfed is still low in many countries.40

Key Charts on Pneumonia
See all charts on this topicEndnotes
We use the term pneumonia here as a broad term for lower respiratory infections, as explained earlier on the page.
Watkins, K., & Sridhar, D. (2018). Pneumonia: a global cause without champions. The Lancet, 392(10149), 718-719.
The Lancet Global Health Editorial (2018). The disgraceful neglect of childhood pneumonia. The Lancet. Global health, 6(12), e1253.
Institute for Health Metrics and Evaluation (IMHE). (2014). Pushing the Pace: Progress and Challenges in Fighting Childhood Pneumonia.
McAllister, D. A., Liu, L., Shi, T., Chu, Y., Reed, C., Burrows, J., ... & Nair, H. (2019). Global, regional, and national estimates of pneumonia morbidity and mortality in children younger than 5 years between 2000 and 2015: a systematic analysis.The Lancet Global Health, 7(1), e47-e57.
Troeger, C., Blacker, B., Khalil, I. A., Rao, P. C., Cao, J., Zimsen, S. R., … & Adetifa, I. M. O. (2018). Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016.The Lancet Infectious Diseases, 18(11), 1191-1210.
Chisti, M. J., Tebruegge, M., La Vincente, S., Graham, S. M., & Duke, T. (2009). Pneumonia in severely malnourished children in developing countries–mortality risk, aetiology and validity of WHO clinical signs: a systematic review. Tropical medicine & international health, 14(10), 1173-1189.
Dherani, M., Pope, D., Mascarenhas, M., Smith, K. R., Weber, M., & Bruce, N. (2008). Indoor air pollution from unprocessed solid fuel use and pneumonia risk in children aged under five years: a systematic review and meta-analysis.
Bulletin of the World Health Organization (2006). Air quality guidelines: global update 2005. p123-124.
Nel, A. (2005). Air pollution-related illness: effects of particles. Science, 308(5723), 804-806.
Öberg, M., Jaakkola, M. S., Woodward, A., Peruga, A., & Prüss-Ustün, A. (2011). Worldwide burden of disease from exposure to second-hand smoke: a retrospective analysis of data from 192 countries. The Lancet, 377(9760), 139-146. The study suggested that exposure to secondhand smoke led to 165,000 deaths among children under 5 from lower respiratory diseases that year.
Theodoratou, E., McAllister, D. A., Reed, C., Adeloye, D. O., Rudan, I., Muhe, L. M., … & Nair, H. (2014). Global, regional, and national estimates of pneumonia burden in HIV-infected children in 2010: a meta-analysis and modelling study. The Lancet Infectious Diseases, 14(12), 1250-1258.
Supplement to: McAllister DA, Liu L, Shi T, et al. Global, regional, and national estimates of pneumonia morbidity and mortality in children younger than 5 years between 2000 and 2015: a systematic analysis. Lancet Glob Health 2018; published online Nov 26.
Lazzerini, M., Sonego, M., & Pellegrin, M. C. (2015). Hypoxaemia as a mortality risk factor in acute lower respiratory infections in children in low and middle-income countries: systematic review and meta-analysis. PLoS One, 10(9), e0136166.
The air we breathe contains 21% of oxygen gas, but it is possible to concentrate this gas using special oxygen concentrators. The oxygen-enriched air can then be supplied to a person with pneumonia via a breathing mask, in this way compensating for reduced oxygen exchange in the lungs.
World Health Organization. (2016). Oxygen therapy for children: a manual for health workers.
World Health Organization. (2019). WHO model list of essential medicines: 7th list, August 2019.
Delarosa, J., Hayes, J., Pantjushenko, E., Keith, B., Ambler, G. and Lawrence, C. (2017). Oxygen Is Essential: A Policy and Advocacy Primer. [online] PATH. [Accessed 5 Sep. 2019].
Troeger, C., Blacker, B., Khalil, I. A., Rao, P. C., Cao, J., Zimsen, S. R., ... & Adetifa, I. M. O. (2018). Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet Infectious Diseases, 18(11), 1191-1210.
Cohen, C., Von Mollendorf, C., De Gouveia, L., Lengana, S., Meiring, S., Quan, V., ... & Madhi, S. A. (2017). Effectiveness of the 13-valent pneumococcal conjugate vaccine against invasive pneumococcal disease in South African children: a case-control study. The Lancet Global Health, 5(3), e359-e369.
Lucero, M. G., Dulalia, V. E., Nillos, L. T., Williams, G., Parreño, R. A. N., Nohynek, H., ... & Makela, H. (2009). Pneumococcal conjugate vaccines for preventing vaccine‐type invasive pneumococcal disease and X‐ray defined pneumonia in children less than two years of age.Cochrane Database of Systematic Reviews, (4).
Moore, M. R., Link-Gelles, R., Schaffner, W., Lynfield, R., Holtzman, C., Harrison, L. H., ... & Thomas, A. (2016). Effectiveness of 13-valent pneumococcal conjugate vaccine for prevention of invasive pneumococcal disease in children in the USA: a matched case-control study.The Lancet Respiratory Medicine, 4(5), 399-406.
Who.int. (2019) — Immunization coverage. [online] [Accessed 10 Sep. 2019]. http://view-hub.org/viz/ (Go to PCV —> PCV - Vaccine Access —> Children without Access)
Hanada, S., Pirzadeh, M., Carver, K. Y., & Deng, J. C. (2018). Respiratory Viral Infection-Induced Microbiome Alterations and Secondary Bacterial Pneumonia.Frontiers in immunology, 9, 2640.
Song, J. Y., Nahm, M. H., & Moseley, M. A. (2013). Clinical implications of pneumococcal serotypes: invasive disease potential, clinical presentations, and antibiotic resistance. Journal of Korean medical science, 28(1), 4-15.
The number of serotypes included in the vaccine is generally indicated in its name, e.g. PCV13 is pneumococcal conjugate vaccine effective against 13 bacterial serotypes. Vaccines including progressively more serotypes have been introduced over the years, PCV7 was introduced in 2000 and today the most commonly used PCV13 was introduced in 2010.
Hausdorff, W. P., Feikin, D. R., & Klugman, K. P. (2005). Epidemiological differences among pneumococcal serotypes.The Lancet infectious diseases, 5(2), 83-93.
The 27% refers to X-ray-defined cases of pneumonia. For clinically defined pneumonia, a less accurate diagnosis than X-ray-defined cases, the number is 6%. Both of these indicators refer to cases of pneumonia caused by any pathogen not only pneumococcus. Lucero, M. G., Dulalia, V. E., Nillos, L. T., Williams, G., Parreño,
R. A. N., Nohynek, H., ... & Makela, H. (2009). Pneumococcal conjugate vaccines for preventing vaccine‐type invasive pneumococcal disease and X‐ray defined pneumonia in children less than two years of age. Cochrane Database of Systematic Reviews, (4).
The current non-conjugate vaccine, PPSV23, is generally only given to adults or as a single dose following two immunisations with PCV13 in children older than 2.
Centers for Disease Control and PRevention (2023). Pneumococcal Vaccination: Summary of Who and When to Vaccinate. Available online.
Golos, M., Eliakim‐Raz, N., Stern, A., Leibovici, L., & Paul, M. (2016). Conjugated pneumococcal vaccine versus polysaccharide pneumococcal vaccine for prevention of pneumonia and invasive pneumococcal disease in immunocompetent and immunocompromised adults and children. Cochrane Database of Systematic Reviews, (8).
Wahl, B., O'Brien, K. L., Greenbaum, A., Majumder, A., Liu, L., Chu, Y., ... & Rudan, I. (2018). Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: global, regional, and national estimates for 2000–15. The Lancet Global Health, 6(7), e744-e757.
Chen, C., Liceras, F. C., Flasche, S., Sidharta, S., Yoong, J., Sundaram, N., & Jit, M. (2019). Effect and cost-effectiveness of pneumococcal conjugate vaccination: a global modelling analysis. The Lancet Global Health, 7(1), e58-e67.
GAVI (Global Alliance for Vaccines and Immunisation) is a non-profit organisation that provides access to vaccination programs for low-income countries by providing financial support and individual expertise.
O'Brien, K. L. (2018). When less is more: how many doses of PCV are enough?. The Lancet Infectious Diseases, 18(2), 127-128.
For example Kenya has recently entered a transition phase during which it will pay a larger and larger portion of the PCV vaccine cost. By 2027 Kenya will have to pay the full $9 price for a three-dose course child vaccination. The 2016 per capita healthcare expenditure in Kenya was around $66 (5% of the GDP), clearly $9 per child is not a trivial cost.
Simonsen, L., van Wijhe, M., & Taylor, R. (2019). Are expensive vaccines the best investment in low-income and middle-income countries?. The Lancet Global Health, 7(5), e548-e549.
Ojal, J., Griffiths, U., Hammitt, L. L., Adetifa, I., Akech, D., Tabu, C., ... & Flasche, S. (2019). Sustaining pneumococcal vaccination after transitioning from Gavi support: a modelling and cost-effectiveness study in Kenya. The Lancet Global Health, 7(5), e644-e654.
The return of investment was estimated for a projected coverage for individual countries for the decade between 2011 and 2020. It means that the economic benefits (as measured by the costs of vaccination program subtracted from the reduced costs of treatment and productivity loss) of using the vaccine are 3 times higher than no vaccine use.
To reduce costs, some countries may also consider switching to a two rather than three dose immunization schedule, but more research on the effectiveness of this schedule in different countries is needed. See O'Brien et al. (2018) reference.
Nakamura, M. M., Tasslimi, A., Lieu, T. A., Levine, O., Knoll, M. D., Russell, L. B., & Sinha, A. (2011). Cost effectiveness of child pneumococcal conjugate vaccination in middle-income countries.International health, 3(4), 270-281.
Ozawa, S., Clark, S., Portnoy, A., Grewal, S., Brenzel, L., & Walker, D. G. (2016). Return on investment from childhood immunization in low-and middle-income countries, 2011–20.Health Affairs, 35(2), 199-207.
Center, I. V. A. (2017). The evidence base for pneumococcal conjugate vaccines (PCVs): data for decision-making around PCV use in childhood.Baltimore (MD): Johns Hopkins University.
Goldblatt, D., Southern, J., Andrews, N. J., Burbidge, P., Partington, J., Roalfe, L., ... & Snape, M. D. (2018). Pneumococcal conjugate vaccine 13 delivered as one primary and one booster dose (1+ 1) compared with two primary doses and a booster (2+ 1) in UK infants: a multicentre, parallel group randomised controlled trial.The Lancet Infectious Diseases, 18(2), 171-179.
O'Brien, K. L. (2018). When less is more: how many doses of PCV are enough?. The Lancet Infectious Diseases, 18(2), 127-128.
Adegbola, R. A., DeAntonio, R., Hill, P. C., Roca, A., Usuf, E., Hoet, B., & Greenwood, B. M. (2014). Carriage of Streptococcus pneumoniae and other respiratory bacterial pathogens in low and lower-middle income countries: a systematic review and meta-analysis. PloS one, 9(8), e103293.
Megiddo, I., Klein, E., & Laxminarayan, R. (2018). Potential impact of introducing the pneumococcal conjugate vaccine into national immunisation programmes: an economic-epidemiological analysis using data from India. BMJ global health, 3(3), e000636.
Johnson, H. L., Deloria-Knoll, M., Levine, O. S., Stoszek, S. K., Hance, L. F., Reithinger, R., ... & O'Brien, K. L. (2010). Systematic evaluation of serotypes causing invasive pneumococcal disease among children under five: the pneumococcal global serotype project.PLoS medicine, 7(10), e1000348.
World Health Organization. (2010). Changing epidemiology of pneumococcal serotypes after introduction of conjugate vaccine: July 2010 report. Weekly Epidemiological Record [Relevé épidémiologique hebdomadaire], 85(43), 434-436.
Pichichero, M. E. (2017). Pneumococcal whole-cell and protein-based vaccines: changing the paradigm. Expert review of vaccines, 16(12), 1181-1190.
Ginsburg, A. S., Nahm, M. H., Khambaty, F. M., & Alderson, M. R. (2012). Issues and challenges in the development of pneumococcal protein vaccines. Expert review of vaccines, 11(3), 279-285
Troeger, C., Blacker, B., Khalil, I. A., Rao, P. C., Cao, J., Zimsen, S. R., ... & Adetifa, I. M. O. (2018). Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016.The Lancet Infectious Diseases, 18(11), 1191-1210.
WHO, U. (2006). Air quality guidelines: global update 2005. p123-124. World Health Organization.
Ferdous, F., Ahmed, S., Das, S. K., Chisti, M. J., Nasrin, D., Kotloff, K. L., ... & Wagatsuma, Y. (2018). Pneumonia mortality and healthcare utilization in young children in rural Bangladesh: a prospective verbal autopsy study.Tropical medicine and health, 46(1), 17.
UNICEF DATA. (2018). Pneumonia in Children. [online] [Accessed 5 Sep. 2019]
World Health Organization. (2014). Revised WHO classification and treatment of pneumonia in children at health facilities: quick reference guide (No. WHO/FWC/MCA/14.9). World Health Organization.
Unicef.org. (2018). Amoxicillin Dispersible Tablets: Market and Supply Update. [online] [Accessed 26 Sep. 2019].
Unicef. (2016). The State of the World's Children 2016. New York: United Nations Children’s Fund.
Lamberti, L. M., Zakarija-Grković, I., Walker, C. L. F., Theodoratou, E., Nair, H., Campbell, H., & Black, R. E. (2013). Breastfeeding for reducing the risk of pneumonia morbidity and mortality in children under two: a systematic literature review and meta-analysis. BMC public health, 13(3), S18.
41% number is estimated by the UNICEF based on the most recent data available for the countries from surveys between 2013-2018.
UNICEF DATA. (2019). Infant and young child feeding. [online][Accessed 4 Sep. 2019].
Cite this work
Our articles and data visualizations rely on work from many different people and organizations. When citing this topic page, please also cite the underlying data sources. This topic page can be cited as:
Bernadeta Dadonaite and Max Roser (2019) - “Pneumonia” Published online at OurWorldinData.org. Retrieved from: 'https://ourworldindata.org/pneumonia' [Online Resource]BibTeX citation
@article{owid-pneumonia,
author = {Bernadeta Dadonaite and Max Roser},
title = {Pneumonia},
journal = {Our World in Data},
year = {2019},
note = {https://ourworldindata.org/pneumonia}
}Reuse this work freely
All visualizations, data, and code produced by Our World in Data are completely open access under the Creative Commons BY license. You have the permission to use, distribute, and reproduce these in any medium, provided the source and authors are credited.
The data produced by third parties and made available by Our World in Data is subject to the license terms from the original third-party authors. We will always indicate the original source of the data in our documentation, so you should always check the license of any such third-party data before use and redistribution.
All of our charts can be embedded in any site.