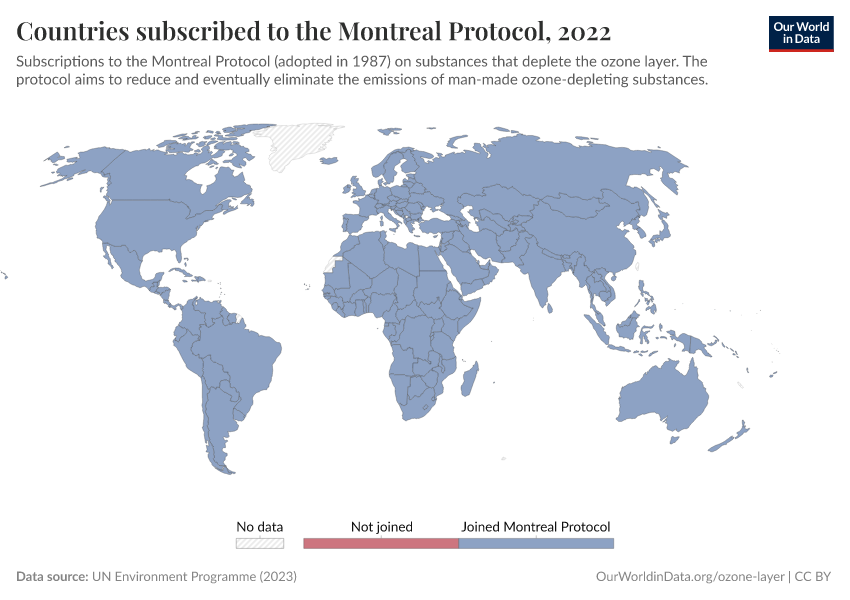
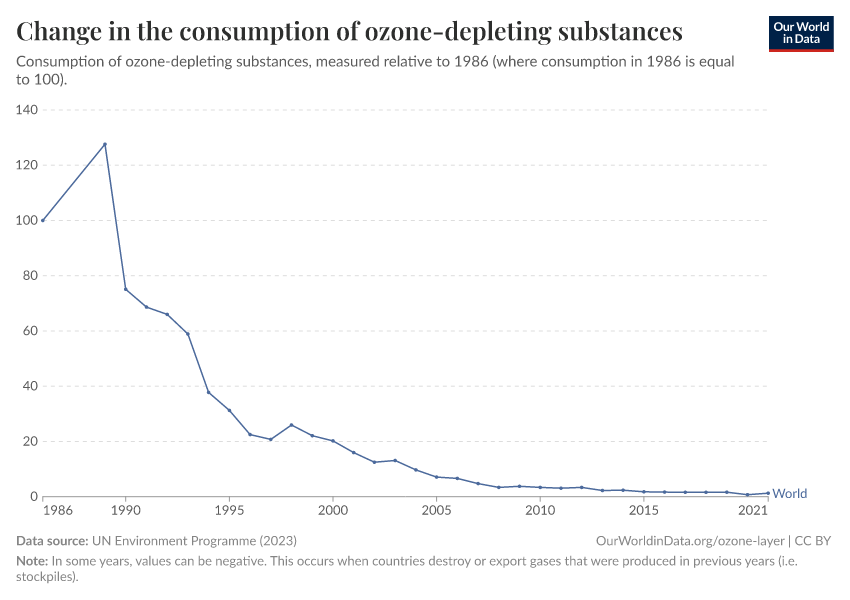
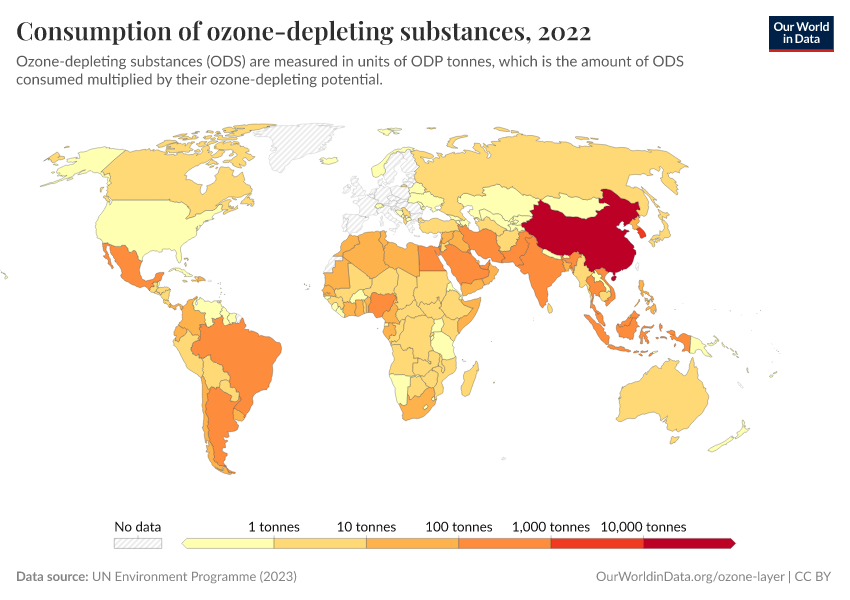
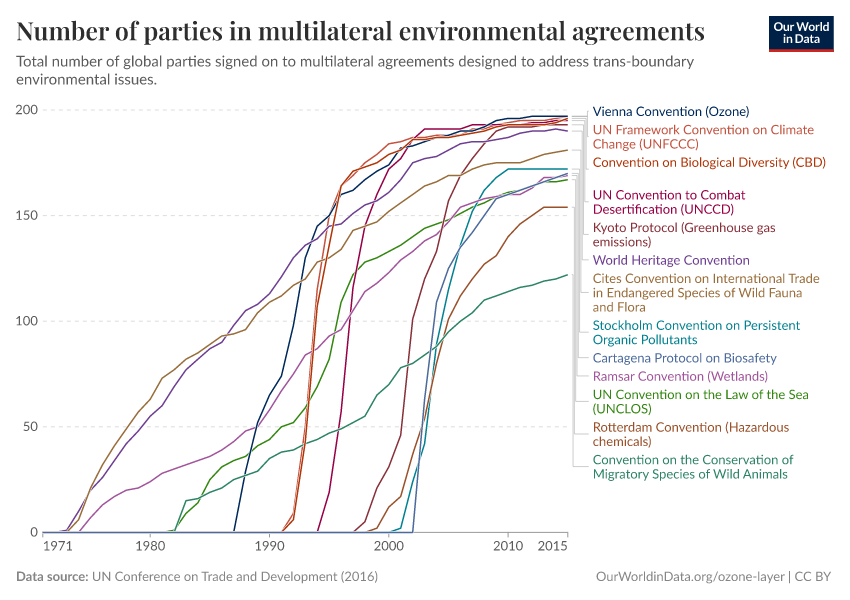
Ozone Layer
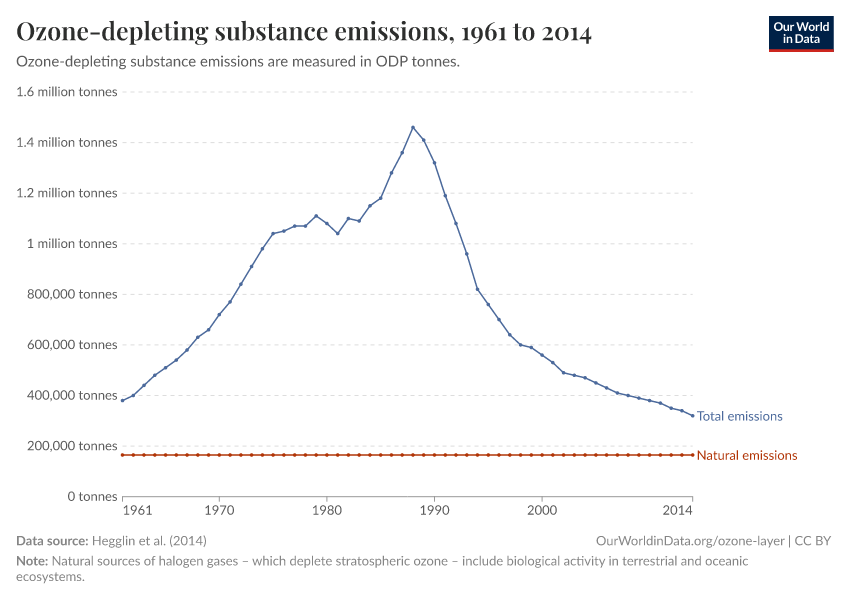
Humans were emitting large amounts of gases that depleted the ozone layer. But in the 1980s the world came together to tackle the problem. Emissions have fallen by more than 99%.
The ozone layer plays a vital role in making the planet habitable for us and other species. High in the atmosphere – between 10 to 50 kilometers above the earth's surface – the ozone layer absorbs most of the sun’s ultraviolet radiation.
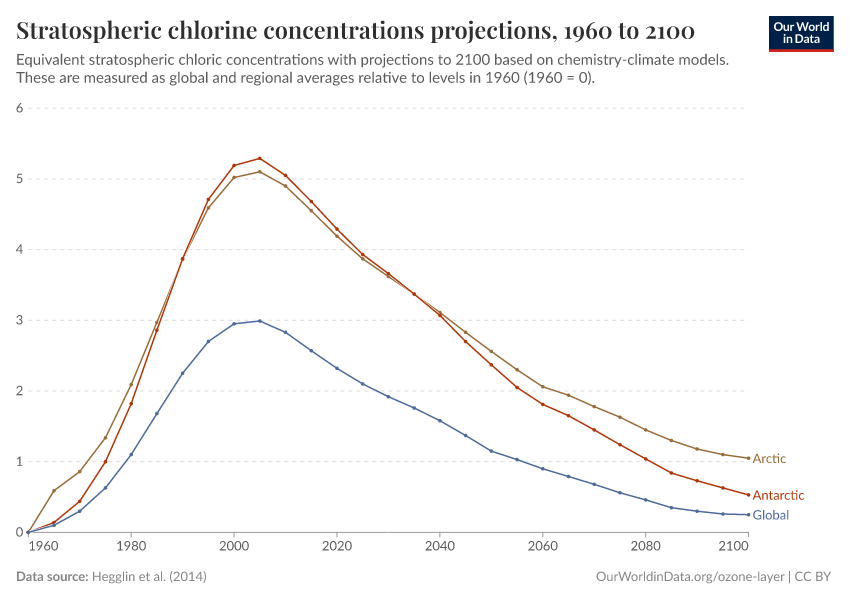
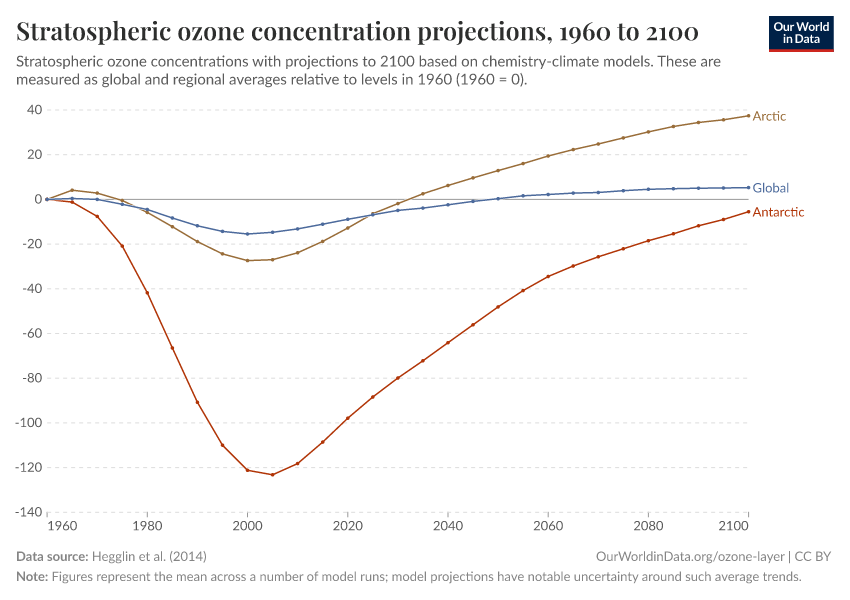
But, during the 1970s, ‘80s, and ‘90s, humans were emitting large quantities of substances that depleted the ozone layer. This led to the creation of ozone holes at the earth’s poles, exposing life to higher levels of ultraviolet radiation and increasing the risks of skin cancer in humans.
During the 1980s, the world came together to form an international agreement to reduce – and eventually eliminate – emissions of these depleting substances. The political agreements were very effective. Since then, global emissions have fallen by more than 99%.
The ozone holes have stopped growing and are now starting to close.
This page includes all of our data, visualizations, and writing on the ozone layer, its depletion, and its path to recovery.
Research & Writing
Key Charts on Ozone Layer
See all charts on this topicEndnotes
Ross J. Salawitch (Lead Author), David W. Fahey, Michaela I. Hegglin, Laura A. McBride, Walter R. Tribett, Sarah J. Doherty, Twenty Questions and Answers About the Ozone Layer: 2018 Update, Scientific Assessment of Ozone Depletion: 2018, 84 pp., World Meteorological Organization, Geneva, Switzerland, 2019.
Strahan, S. E., & Douglass, A. R. (2018). Decline in Antarctic ozone depletion and lower stratospheric chlorine determined from Aura Microwave Limb Sounder observations. Geophysical Research Letters, 45(1), 382-390.
Hegglin, M. I. et al. (2015). Twenty Questions and Answers about the Ozone Layer 2014 Update: Scientific Assessment of Ozone Depletion 2014. World Meteorological Organisation.
Cite this work
Our articles and data visualizations rely on work from many different people and organizations. When citing this topic page, please also cite the underlying data sources. This topic page can be cited as:
Hannah Ritchie, Lucas Rodés-Guirao, and Max Roser (2023) - “Ozone Layer” Published online at OurWorldinData.org. Retrieved from: 'https://ourworldindata.org/ozone-layer' [Online Resource]BibTeX citation
@article{owid-ozone-layer,
author = {Hannah Ritchie and Lucas Rodés-Guirao and Max Roser},
title = {Ozone Layer},
journal = {Our World in Data},
year = {2023},
note = {https://ourworldindata.org/ozone-layer}
}Reuse this work freely
All visualizations, data, and code produced by Our World in Data are completely open access under the Creative Commons BY license. You have the permission to use, distribute, and reproduce these in any medium, provided the source and authors are credited.
The data produced by third parties and made available by Our World in Data is subject to the license terms from the original third-party authors. We will always indicate the original source of the data in our documentation, so you should always check the license of any such third-party data before use and redistribution.
All of our charts can be embedded in any site.