What were the death tolls from pandemics in history?
Pandemics have killed millions of people throughout history. How many deaths were caused by different pandemics, and how have researchers estimated their death tolls?
COVID-19 has brought the reality of pandemics to the forefront of public consciousness. But pandemics have afflicted humanity for millennia. Time and again, people faced outbreaks of diseases — including influenza, cholera, bubonic plague, smallpox, and measles — that spread far and caused death and devastation.
Our ancestors were largely powerless against these diseases and unable to evaluate their true toll on the population. Without good record-keeping of the number of cases and deaths, the impact of outbreaks was underrecognized or even forgotten. The result is that we tend to underestimate the frequency and severity of pandemics in history.
Often, we have records of epidemics occurring in some countries but lack good records from other regions despite knowing that the geographical impact of the disease would have been very wide. Additionally, we often lack knowledge about which pathogens caused outbreaks and, thus, if a historical event can be considered a pandemic or if it consisted of parallel outbreaks of different diseases.
To deal with the lack of historical records on total death tolls, modern historians, epidemiologists, and demographic researchers have used various sources and methods to estimate their death tolls — such as using data from death records, tax registers, land use, archaeological records, epidemiological modeling, and more.
In this article, I present the various methods they rely on and visualize the estimated impact of what are now considered the major pandemics in history.
What is a pandemic?
Although there is no universally accepted definition of a pandemic1, diseases called pandemics share several characteristics.
Pandemics generally refer to diseases with a vast geographic range — such as spreading across a continent or multiple continents. In addition, they tend to describe outbreaks that are rapidly growing or expanding in range, highly infectious, affecting a large number of people, and caused by novel pathogens against which there is little or no pre-existing immunity.2
How do researchers estimate the death toll of pandemics?
Researchers have estimated the death tolls of pandemics in different ways, depending on the data available.
Some death tolls have been estimated by looking at excess deaths: researchers estimate the additional number of deaths that occurred during a pandemic compared to the expected number of deaths in a typical year. This can be helpful to understand the pandemic’s overall impact, even if records from death certificates are unavailable.
For some pandemics, death tolls are estimated from the net population reduction, where researchers calculate the difference in population size before and after the pandemic. This is often used for severe events — such as the Columbian Exchange — where a significant fraction of the population died.
Some death tolls have been estimated through epidemiological modeling — based on knowledge of the transmission of the disease and its geographical spread, its fatality rate (the share of people affected who die from it), access to treatment, and other types of data.
Finally, some death tolls have been calculated only using recorded deaths (also referred to as ‘confirmed deaths’). This is the number of deaths officially reported with the disease as their cause of death. This method may vastly underestimate the number of deaths caused by the pandemic, as comprehensive historical records are lacking. Even today, cause-of-death registration is lacking in many parts of the world, which is one reason why the number of confirmed deaths from COVID-19 is much lower than the total death toll from the pandemic.
A timeline of historical pandemics
I have brought together estimates of death tolls from different pandemics in history for this article, which we have visualized in a timeline below.
The size of each circle represents one pandemic’s estimated death toll. Pandemics without a known death toll are depicted with triangles.
This overview shows us the vast impact that pandemics have had over history.
You can see that the largest pandemics — such as the Black Death — killed more than half of the population. Several pandemics have swept through the population repeatedly: in just the last two hundred years, seven major pandemics were caused by cholera, and another seven were caused by the flu.
Pandemics devastated millions and left a shadow on those who survived. The suffering they caused may once have felt inescapable.
Before the formation of germ theory, we lacked good knowledge of pathogens that caused them, how they spread, and how to protect ourselves from them. Before molecular testing to analyze pathogens’ genomes, we lacked a good understanding of how they evolved and changed over time.
Our ability to respond to pandemics has been transformed by advances in scientific understanding but truly depends on a wide range of efforts — from data collection to research and communication, public health efforts, healthcare access, and cooperation.
For example, the collection of death records allowed scientists to discover how cholera spread and how to prevent it. Coordination to address HIV/AIDS has prevented millions of deaths worldwide. Global testing for new influenza strains has helped adapt flu vaccines each year.
With better understanding, resources, and effort, much more progress can be made. The world can respond more swiftly and effectively to pandemic risks and avoid and reduce the impact of future pandemics. But without such efforts, we will continue to face major pandemics as we have experienced so far.
Dataset and sources
The full dataset and sources used in the chart can be found in our spreadsheet.
In the appendix below, I review some of the major pandemics in history and their historical impact and describe how their death tolls have been estimated.
Appendix: Major pandemics in the last millennium
The Black Death
The Black Death (1346–1353 CE) — one of the earliest pandemics with a methodically estimated death toll — killed around 50–60% of Europe’s population, approximately 50 million people, in just 6 years.3
Researchers have established that many people also died elsewhere — as large outbreaks are also recognizable in historical records from Western Asia, the Middle East, and North Africa — but comprehensive estimates of the global death toll are not available.
Population censuses were not conducted then, so our understanding of the Black Death’s impact in Europe comes from historical records such as tax and rent registers, parish records, and archeological remains. However, uncertainty remains, as these records come from a limited number of European regions and are extrapolated to the rest of the continent based on demographic estimates.
Careful examination of these sources has led to historians revising estimates of the death toll upwards4 and confirmed the bacterial cause of the pandemic: Yersinia pestis (Y. pestis).5
People in the fourteenth century were unaware of this bacterium, nor did they know how it was transmitted — from rat fleas to humans — as this was long before the development of germ theory in the late nineteenth century.6
Without this knowledge, they also had little understanding of how to protect themselves, resulting in the relentless spread of the Black Death. Even after its initial wave, the pandemic continued with frequent, though smaller outbreaks until around 1690.7
Y. pestis caused diseases known as “bubonic” and “pneumonic” plague, where patients experienced fevers, chills, vomiting, and excruciating headaches, and distinctive “buboes” formed in their swollen lymph nodes — typically in the groin, thighs, armpits, or neck.
As Y. pestis spreads through the lymph nodes, it emits toxins that break down blood vessels and form clots, potentially blocking blood circulation and leading to death.8
Other bubonic plague pandemics
It is now recognized that the Black Death was not the only plague caused by Y. pestis. Genetic evidence suggests that this bacteria emerged at least 4000 years ago.9
The first known bubonic plague pandemic began in 541 CE and had recurrent outbreaks until the mid-8th century. This devastating pandemic affected the Eastern Roman Empire (Byzantine Empire), the Middle East, North Africa, and the Mediterranean.
The initial and most severe outbreak, known as the “Justinianic Plague” (541–549 CE), was named after Emperor Justinian, who ruled Constantinople then.10
The third pandemic occurred between 1894 and 1940, mainly affecting Asia and Africa.
Bubonic plague is less common today due to improved sanitation and hygiene measures which reduce the density of rats and rat fleas, improved public health surveillance, and effective antibiotics, but cases have been seen even in recent years in different continents, mainly in small towns and villages.11
The Columbian Exchange
The chart shows the immense impact of the “Columbian Exchange”, with an estimated 48 million deaths.
The Columbian Exchange describes the period following Christopher Columbus’s voyage to the Americas in 1492 — during which populations, ideas, and crops, such as tomatoes, potatoes, and maize, spread between the Americas and the rest of the world.
But the Columbian Exchange also involved extensive war, conquest, slavery, and the spread of multiple deadly diseases, which led to the devastation of indigenous populations.12
Smallpox, cholera, measles, diphtheria, influenza, typhoid fever, bubonic plague, and other diseases had already killed many in Europe. But they tended to be more severe to Native Americans, who had been previously isolated from these diseases and lacked immunity to them.
The immense death toll shown on the chart is calculated as a “net population reduction” compared to the pre-1492 population size.
The Native American population was estimated to be around 54 million before Columbus’s arrival. Over the following century, around 48 million died, and the population declined to 5.6 million in 1600 — a reduction of about 90%.
Both numbers are estimated by compiling data from a range of sources, including archeological records, tribal records, censuses, epidemiological modeling, and land and crop use.13
Influenza pandemics
Influenza (flu) pandemics arise through sudden evolutionary changes in flu viruses when different strains combine to form novel flu strains14, which can be more infectious and lethal than previous ones.
Although flu has affected humanity for thousands of years15, comprehensive death tolls have only been estimated for the flu pandemics in the last 140 years.
The largest — the 1918 “Spanish flu” pandemic — has an estimated death toll of 50 to 100 million.16 This estimate is a compilation of various historical sources, including recorded death tolls and estimates of excess deaths from different regions.
You can read more about the impact of the Spanish flu pandemic in our article:
The chart also shows the estimated impact of other significant flu pandemics: the 1889 “Russian flu” pandemic (an estimated 4 million deaths), the 1957 “Asian flu” pandemic (2 million), the 1968 “Hong Kong flu” pandemic (2 million), and the 2009 “Swine flu” pandemic (100,000 to 1.9 million deaths).17
Their death tolls have been estimated from excess mortality during pandemics compared to the years immediately before and after, using available national mortality records and extrapolation to the global population.
Cholera pandemics
As the chart shows, seven cholera pandemics have occurred in the last two centuries.18 Most are considered to have originated in the Indian subcontinent and expanded across countries and continents through war, travel, and international trade.19
Our knowledge of the total global death toll from cholera in history is limited20, but historical reports from across the world suggest an immense impact of the disease. For example, between 1865 and 1947, at least 23 million people died from cholera in India alone.21 But significant outbreaks have been recorded in many more countries.22
Cholera is particularly severe because, if left untreated, the bacteria Vibrio cholerae can cause severe dehydration and death within hours or days of the first symptoms.23
Its severity has been reduced with a range of scientific advances: the understanding that cholera spread through contaminated water and food, and thus that clean water and sanitation could prevent it; the identification of Vibrio cholerae as the cause; the development of antibiotics; and the knowledge that severe forms of the disease could be substantially reduced with simple rehydration treatment.24
Cholera continues to kill, even today. Since 1960, over 900,000 deaths have been recorded from cholera globally as part of what’s considered the “seventh cholera pandemic” — this is shown in the chart.25
HIV/AIDS
When HIV/AIDS (acquired immunodeficiency syndrome) was first identified in the early 1980s, it had a fatality rate of 100%, and patients had a median survival time of about one year after being diagnosed.26 It spread rapidly as the world grappled with recognizing, understanding, and responding to the growing epidemic.
HIV, the virus that causes AIDS, attacks white blood cells — which are critical for our immune function — and leaves patients vulnerable to a wide range of opportunistic infections and diseases.
Learn more on our page on HIV/AIDS:
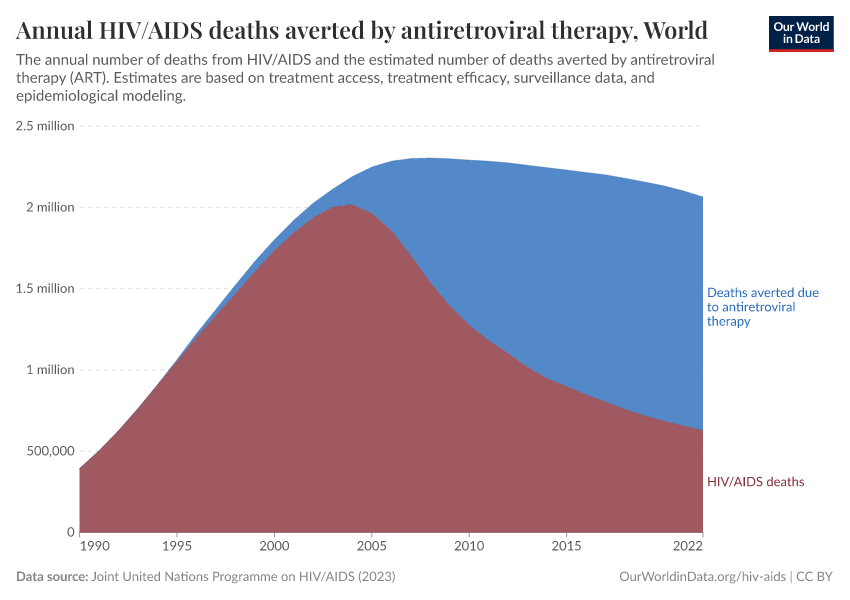
The timeline shows the enormous and continuing impact of HIV/AIDS, which has resulted in an estimated 33 million deaths worldwide between 1981 and 2022.
Our understanding of its death toll comes from available data and statistical modeling. The estimates consider various factors, such as characteristics of the virus’s transmission, behavioral and clinical data, the availability of treatment, and recorded deaths from countries with high levels of death registration. 27
The global response to HIV/AIDS has involved international cooperation, resource allocation, and scientific advances in antiretroviral therapy, which together have transformed HIV from a fatal diagnosis to a manageable chronic condition with treatment.
In recent years, around 1.5 million deaths have been averted annually due to the effects of antiretroviral therapy — which prevents the virus from replicating and thereby reduces the severity of the disease and its spread to other individuals.
This is shown in the chart below, along with the estimated number of HIV/AIDS deaths that still occur — around 600,000 deaths annually in recent years.
COVID-19
The COVID-19 pandemic was caused by the novel coronavirus SARS-CoV-2, which emerged at the end of 2019 and rapidly evolved into a global health emergency. Characterized by its highly infectious nature and severe respiratory symptoms, COVID-19 has led to widespread illness and fatalities across the world.
The timeline above shows the vast global impact of the COVID-19 pandemic — with around 27 million excess deaths between January 2020 and November 2023.28 This makes it one of the deadliest pandemics of the last century.
COVID-19’s death toll has been measured by excess mortality, which describes the number of deaths above what would have been expected based on previous years.
This method is used because the global number of confirmed deaths from COVID-19 (those where COVID-19 is listed as the cause of death) is certainly much lower than the total number of deaths from COVID-19. This is because, in many countries, testing for COVID was very limited throughout the pandemic, and cause-of-death registration was, and still is, lacking in many countries.29
Excess mortality also has the advantage of not only considering deaths directly caused by the virus but also those indirectly caused by the pandemic’s impact on healthcare systems and economies.
Learn more about excess mortality here:
To estimate excess mortality, researchers use national mortality data from countries where data is available and statistical models — which rely on data on COVID-19 testing rates, confirmed cases and deaths, population age structure, state policies, and more — for other countries.
The estimated death toll we show for COVID-19 — 27 million deaths by November 2023 — comes from The Economist. The main reasons why I am relying on The Economist’s estimates are that they are continuously updated, and their methodology is well documented.
In contrast, while the World Health Organization (WHO) and the Institute for Health Metrics and Evaluation (IHME) have also estimated the number of excess deaths, their latest estimates were only based on the time period until the end of 2021. For this time period, all three sources provide similar estimates (18.2 million deaths were estimated by the IHME; 14.8 million deaths were estimated by the WHO; and 17.8 million deaths were estimated by The Economist).30
The chart below shows the excess mortality during COVID-19, as estimated by The Economist, along with the number of confirmed deaths. As you can see, there is wide uncertainty around the total number of excess deaths during the pandemic. However, even the lowest estimates are much higher than the number of confirmed deaths — reflecting the limited amount of testing and death registration globally during the pandemic.

Endnotes
The World Health Organization (WHO) defines pandemics in the context of influenza pandemics. They are defined as occurring ‘when key factors converge: a novel influenza virus emerges that has the ability to cause sustained human-to-human transmission, the virus causes disease, and the human population has little to no immunity against the virus.’
However, there is no official definition of a pandemic for diseases in general.
Instead, the highest outbreak response designated by the WHO is the ‘Public Health Emergency of International Concern (PHEIC)’, a formal declaration involving an official institutional process.
A PHEIC is used to designate ‘an extraordinary event which is determined to constitute a public health risk to other States through the international spread of disease and to potentially require a coordinated international response’, and it carries a legal obligation for countries to respond promptly to the outbreak.
For more discussion, see also:
World Health Organization. (2017). WHO guidance for surveillance during an influenza pandemic. https://iris.who.int/bitstream/handle/10665/259886/9789241513333-eng.pdf
Emma Ross. (2022). ‘What is the difference between a pandemic and a PHEIC?’ Chatham House.
https: //www.chathamhouse.org/2022/10/what-difference-between-pandemic-and-pheic
Wilder-Smith, A., & Osman, S. (2020). Public health emergencies of international concern: A historic overview. Journal of Travel Medicine, 27(8), taaa227. https://doi.org/10.1093/jtm/taaa227
University of the Witswatersrand. (2023). Future-proofing pandemic preparedness and response: A comparative analysis of the CA+ and IHR amendments. https://www.wits.ac.za/media/wits-university/news-and-events/images/documents/2023/Future-proofing%20pandemic%20preparedness%20and%20response.pdf
Singer, B. J., Thompson, R. N., & Bonsall, M. B. (2021). The effect of the definition of ‘pandemic’ on quantitative assessments of infectious disease outbreak risk. Scientific Reports, 11(1), 2547. https://doi.org/10.1038/s41598-021-81814-3
Morens, D. M., Folkers, G. K., & Fauci, A. S. (2009). What Is a Pandemic? The Journal of Infectious Diseases, 200(7), 1018–1021. https://doi.org/10.1086/644537
Morens, D. M., Folkers, G. K., & Fauci, A. S. (2009). What Is a Pandemic? The Journal of Infectious Diseases, 200(7), 1018–1021. https://doi.org/10.1086/644537
Benedictow, O. J. (2021). The Complete History of the Black Death. Boydell & Brewer.
Aberth, J. (2021). The Black Death: A new history of the great mortality in Europe, 1347-1500. Oxford University Press.
Benedictow estimates that upwards of 60% of the population of Europe died during the Black Death, while Aberth estimates around 51–58%.
Both compile a vast collection of parish records, tax and court documents, historical reviews, hospital records, and archeological remains to come to these overall estimates. Many records they use are newly digitized and unarchived. They also explain why previous estimates from historians in the 20th century were too low — aside from lacking sources that have been unarchived more recently, they point out that previous records came disproportionately from the nobility, who tended to have better nutrition and hygiene and likely lower fatality rates.
In addition, they explain why rural populations were not spared from the pandemic — as rats and fleas, which were vectors for Yersinia pestis, were abundant in both small, rural villages and crowded urban areas.
Despite these thorough historiographic estimates, there is remaining uncertainty around the death toll.
First, deaths estimated through this method are based on the net change in mortality during the pandemic. This means they can include other events during that time and do not account for declines in other sources of death.
Second, available records are limited to Europe, and specifically parts of Europe — such as regions of Spain, Italy, France, and England. Hundreds of communities have been studied within these geographies, ranging from hamlets to towns, villages, and cities. Estimates from these regions are used to estimate the death toll across Europe based on demographic estimates of the population size and structure.
Benedictow concludes that available sources largely come to a consistent conclusion that upwards of 60% of Europe’s population died during the Black Death, although other sources such as Christakos et al. and Aberth put the estimate lower, at 40% and 51–58%, respectively.
In their working paper, Jedwab, Johnson, and Koyama (2019) estimate that 39% of Western Europe’s population died based on geospatial extrapolation of data from Christakos et al. (2005), which compiled various historical records.
Here, I have given a rounded estimate of 50–60%, using the recent and comprehensive studies by Aberth and Benedictow.
Jedwab, R., Johnson, N. D., & Koyama, M. (2020). Pandemics and Cities: Evidence from the Black Death and the Long-Run. Available at SSRN 4181983.
Christakos et al. (2005). Interdisciplinary Public Health Reasoning and Epidemic Modelling: The Case of Black Death. Springer Berlin Heidelberg. https://doi.org/10.1007/3-540-28165-7
Alfani, G., & Murphy, T. E. (2017). Plague and Lethal Epidemics in the Pre-Industrial World. The Journal of Economic History, 77(1), 314–343. https://doi.org/10.1017/S0022050717000092
Spyrou, M. A., Tukhbatova, R. I., Feldman, M., Drath, J., Kacki, S., Beltrán de Heredia, J., Arnold, S., Sitdikov, A. G., Castex, D., Wahl, J., Gazimzyanov, I. R., Nurgaliev, D. K., Herbig, A., Bos, K. I., & Krause, J. (2016). Historical Y. pestis Genomes Reveal the European Black Death as the Source of Ancient and Modern Plague Pandemics. Cell Host & Microbe, 19(6), 874–881. https://doi.org/10.1016/j.chom.2016.05.012
Y. pestis bacteria prefer to infect rat colonies first, and when these colonies die off, they turn to humans nearby.
Hinnebusch, B. J., Chouikha, I., & Sun, Y.-C. (2016). Ecological Opportunity, Evolution, and the Emergence of Flea-Borne Plague. Infection and Immunity, 84(7), 1932–1940. https://doi.org/10.1128/IAI.00188-16
Benedictow, O. J. (2021). The Complete History of the Black Death. Boydell & Brewer.
Benedictow, O. J. (2021). The Complete History of the Black Death. Boydell & Brewer.
Aberth, J. (2021). The Black Death: A new history of the great mortality in Europe, 1347-1500. Oxford University Press.
Wiechmann, I., Benedictow, O. J., Bianucci, R., & Kacki, S. (2012). History of the plague. RCC Perspectives, 3, 63–74. https://www.jstor.org/stable/26242596
Spyrou, M. A., Tukhbatova, R. I., Wang, C.-C., Valtueña, A. A., Lankapalli, A. K., Kondrashin, V. V., Tsybin, V. A., Khokhlov, A., Kühnert, D., Herbig, A., Bos, K. I., & Krause, J. (2018). Analysis of 3800-year-old Yersinia pestis genomes suggests Bronze Age origin for bubonic plague. Nature Communications, 9(1), 2234. https://doi.org/10.1038/s41467-018-04550-9
Benedictow, O. J. (2017). Plague, Historical. In International Encyclopedia of Public Health (pp. 473–488). Elsevier. https://doi.org/10.1016/B978-0-12-803678-5.00332-5
Centers for Disease Control and Prevention. (2022). Plague: Maps and Statistics. https://www.cdc.gov/plague/maps/index.html
World Health Organization. (2022, July). Plague: Fact sheet. https://www.who.int/news-room/fact-sheets/detail/plague
Bramanti, B., Dean, K. R., Walløe, L., & Chr. Stenseth, N. (2019). The Third Plague Pandemic in Europe. Proceedings of the Royal Society B: Biological Sciences, 286(1901), 20182429. https://doi.org/10.1098/rspb.2018.2429
Echenberg, M. J. (2002). Pestis Redux: The Initial Years of the Third Bubonic Plague Pandemic, 1894-1901. Journal of World History, 13(2), 429–449. https://doi.org/10.1353/jwh.2002.0033
Wiechmann, I., Benedictow, O. J., Bianucci, R., & Kacki, S. (2012). History of the plague. RCC Perspectives, 3, 63–74. https://www.jstor.org/stable/26242596
Nunn, N., & Qian, N. (2010). The Columbian Exchange: A History of Disease, Food, and Ideas. Journal of Economic Perspectives, 24(2), 163–188. https://doi.org/10.1257/jep.24.2.163
Estimates for the pre-Columbian population of the Americas, based on historical records and censuses, are generally uncertain because there was a large initial death toll from conquest and disease, because diseases may have spread faster and further than Europeans encountered those communities, and because of a lack of remaining historical records.
Recently, archeological evidence has also been used to make estimates of land use, which can then help develop and improve estimates of population size. These have led to revising estimates of the initial population size upwards. They also depend on assumptions about various factors, such as the number of houses in settlements occupied by inhabitants at a given time, for example.
The estimates have relied on many data sources and important assumptions. Here, I cite figures by Koch et al. (2019), which agree with other recent estimates by HYDE (Klein et al. 2017), also based on land use, and are similar to previous estimates by Thornton (1990), based on historical death records and epidemiological modeling.
Koch, A., Brierley, C., Maslin, M. M., & Lewis, S. L. (2019). Earth system impacts of the European arrival and Great Dying in the Americas after 1492. Quaternary Science Reviews, 207, 13–36. https://doi.org/10.1016/j.quascirev.2018.12.004
Klein Goldewijk, K., Beusen, A., Doelman, J., & Stehfest, E. (2017). Anthropogenic land use estimates for the Holocene – HYDE 3.2. Earth System Science Data, 9(2), 927–953. https://doi.org/10.5194/essd-9-927-2017
For further reading on the spread and impact of disease in the Americas, see also:
Cook, N. D. (1999). Born to die: Disease and New World conquest, 1492 - 1650 (Reprinted 1998). Cambridge Univ. Press.
Thornton, R. (1990). American Indian holocaust and Survival ; a Population History Since 1492. Univ. of Oklahoma Press.
Thornton, R., Miller, T., & Warren, J. (1991). American Indian Population Recovery Following Smallpox Epidemics. American Anthropologist, 93(1), 28–45. https://doi.org/10.1525/aa.1991.93.1.02a00020
This process is known as antigenic shift.
Mamelund, S.-E. (2008). Influenza, historical. Medicine, 54, 361–371.
Johnson and Mueller arrive at an estimate of 50 million by compiling a wide range of different mortality records, including vital registration data, excess mortality estimates, and previous historical estimates for specific regions.
They explain that there is substantial uncertainty in these estimates due to a lack of death registration in many countries, missing records, misdiagnosis, and a tendency towards underreporting deaths in rural or native populations, and thus state that the true death toll may be up to 100 million.
Before this estimate, historians had previously estimated a range of figures: from 30 million deaths globally (Patterson and Pyle, 1991) to 18 million in India alone (Mills, 1986).
Recent research (Spreeuwenberg et al., 2018) has estimated a global death toll of around 17 million, using excess mortality estimates from high-income countries with high levels of death registration and extrapolating mortality rates to the global population. However, many sources have found higher flu mortality rates in poorer countries, suggesting this is likely underestimated.
Johnson, N. P., & Mueller, J. (2002). Updating the accounts: Global mortality of the 1918-1920" Spanish" influenza pandemic. Bulletin of the History of Medicine, 105–115.
Patterson, K. D., & Pyle, G. F. (1991). The geography and mortality of the 1918 influenza pandemic. Bulletin of the History of Medicine, 65(1), 4–21.
Spreeuwenberg, P., Kroneman, M., & Paget, J. (2018). Reassessing the Global Mortality Burden of the 1918 Influenza Pandemic. American Journal of Epidemiology, 187(12), 2561–2567. https://doi.org/10.1093/aje/kwy191
McLane, J. R. (2013). Paradise locked: The 1918 influenza pandemic in American Samoa. Sites: a journal of social anthropology and cultural studies, 10(2), 30-51.
Mamelund, S. E. (2017). Profiling a Pandemic. Who were the victims of the Spanish flu?
The names of flu pandemics don’t necessarily describe where each pandemic originated but tend to describe where they were first reported.
Kaper, J. B., Morris, J. G., & Levine, M. M. (1995). Cholera. Clinical Microbiology Reviews, 8(1), 48–86. https://doi.org/10.1128/CMR.8.1.48
Historically, this took place during British rule of India, and the disease was spread partly through the travel of soldiers and troops.
It’s believed that the endemicity of cholera in the Indian subcontinent is related to the climate around the Bay of Bengal.
The seventh cholera pandemic is considered to have originated on the island of Sulawesi in Indonesia. Still, genetic research has shown that numerous waves emerged from the Indian subcontinent and spread across countries.
This seventh pandemic was caused by the El Tor biotype, which is different from the ‘classic biotype’ that caused all previous pandemics.
Mutreja, A., Kim, D. W., Thomson, N. R., Connor, T. R., Lee, J. H., Kariuki, S., Croucher, N. J., Choi, S. Y., Harris, S. R., Lebens, M., Niyogi, S. K., Kim, E. J., Ramamurthy, T., Chun, J., Wood, J. L. N., Clemens, J. D., Czerkinsky, C., Nair, G. B., Holmgren, J., … Dougan, G. (2011). Evidence for several waves of global transmission in the seventh cholera pandemic. Nature, 477(7365), 462–465. https://doi.org/10.1038/nature10392
Richard, A. L., & DiRita, V. J. (2013). Cholera. In E. Rosenberg, E. F. DeLong, S. Lory, E. Stackebrandt, & F. Thompson (Eds.), The Prokaryotes (pp. 125–131). Springer Berlin Heidelberg. https://doi.org/10.1007/978-3-642-30144-5_92
Until the 1860s, the collection of vital statistics had not begun in India and was also lacking in other affected countries.
This estimate comes largely from vital registration data from the time — official death records collected across British India — and is likely to underestimate the actual death toll due to under-recording, missing records, and coverage.
Vital registration was instituted across India in the 1870s, and estimates before that come from extrapolating mortality rates from some regions, such as Bengal, Bombay islands, and Madras, to the rest of India.
Arnold, D. (1986). Cholera and colonialism in British India. Past and Present, 113(1), 118–151. https://doi.org/10.1093/past/113.1.118
Echenberg, M. (2011). Africa in the Time of Cholera: A History of Pandemics from 1817 to the Present. Cambridge University Press.
Kotar, S. L., & Gessler, J. E. (2014). Cholera: A worldwide history. McFarland & Company, Inc., Publishers.
Chan, C. H., Tuite, A. R., & Fisman, D. N. (2013). Historical Epidemiology of the Second Cholera Pandemic: Relevance to Present Day Disease Dynamics. PLoS ONE, 8(8), e72498. https://doi.org/10.1371/journal.pone.0072498
Kanungo, S., Azman, A. S., Ramamurthy, T., Deen, J., & Dutta, S. (2022). Cholera. The Lancet, 399(10333), 1429–1440. https://doi.org/10.1016/S0140-6736(22)00330-0
During large outbreaks in the 19th century, between 30 and 60% of people diagnosed with cholera died from it in many major cities, including New York and Berlin.
The case-fatality rate of cholera now ranges between 0 to 10% globally. However, this figure has limitations: many countries lack surveillance and reporting of cholera cases. Furthermore, some countries use different definitions of cases due to a lack of testing capacity. For example, some countries describe all cases of watery diarrhea as cholera cases during outbreaks due to limited laboratory capacity.
Chan, C. H., Tuite, A. R., & Fisman, D. N. (2013). Historical Epidemiology of the Second Cholera Pandemic: Relevance to Present Day Disease Dynamics. PLoS ONE, 8(8), e72498. https://doi.org/10.1371/journal.pone.0072498
Kanungo, S., Azman, A. S., Ramamurthy, T., Deen, J., & Dutta, S. (2022). Cholera. The Lancet, 399(10333), 1429–1440. https://doi.org/10.1016/S0140-6736(22)00330-0
Deen, J., Mengel, M. A., & Clemens, J. D. (2020). Epidemiology of cholera. Vaccine, 38, A31–A40. https://doi.org/10.1016/j.vaccine.2019.07.078
Santosham, M., Duggan, C. P., & Glass, R. (2019). Elimination of diarrheal mortality in children – the last half million. Journal of Global Health, 9(2), 020102. https://doi.org/10.7189/jogh.09.020102
Bhattacharya, S. K. (1994). History of development of oral rehydration therapy. Indian Journal of Public Health, 38(2), 39–43. https://pubmed.ncbi.nlm.nih.gov/7530695/
Binder, H. J., Brown, I., Ramakrishna, B. S., & Young, G. P. (2014). Oral Rehydration Therapy in the Second Decade of the Twenty-first Century. Current Gastroenterology Reports, 16(3), 376. https://doi.org/10.1007/s11894-014-0376-2
This is the cumulative total of deaths reported to the World Health Organization between 1960 and 2021.
World Health Organization; compiled by Our World in Data (2022). Number of reported deaths from cholera. Available online: https://ourworldindata.org/grapher/number-of-reported-cholera-deaths
Ganesan, D., Gupta, S. S., & Legros, D. (2020). Cholera surveillance and estimation of burden of cholera. Vaccine, 38, A13–A17. https://doi.org/10.1016/j.vaccine.2019.07.036
Wiens, K. E., Xu, H., Zou, K., Mwaba, J., Lessler, J., Malembaka, E. B., Demby, M. N., Bwire, G., Qadri, F., Lee, E. C., & Azman, A. S. (2022). Towards estimating true cholera burden: A systematic review and meta-analysis of Vibrio cholerae positivity [Preprint]. MedRxiv. https://doi.org/10.1101/2022.10.05.22280736
Bacchetti, P., Osmond, D., Chaisson, R. E., Dritz, S., Rutherford, G. W., Swig, L., & Moss, A. R. (1988). Survival patterns of the first 500 patients with AIDS in San Francisco. The Journal of Infectious Diseases, 157(5), 1044–1047. https://www.nejm.org/doi/full/10.1056/NEJM198711193172101
Rothenberg, R., Woelfel, M., Stoneburner, R., Milberg, J., Parker, R., & Truman, B. (1987). Survival with the Acquired Immunodeficiency Syndrome. New England Journal of Medicine, 317(21), 1297–1302. https://doi.org/10.1056/NEJM198711193172101
Mahy, M., Marsh, K., Sabin, K., Wanyeki, I., Daher, J., & Ghys, P. D. (2019). HIV estimates through 2018: Data for decision-making. AIDS (London, England), 33 Suppl 3(Suppl 3), S203–S211. https://doi.org/10.1097/QAD.0000000000002321
UNAIDS. (2022a). Global HIV statistics. https://web.archive.org/web/20231019185539/https://www.aidsdatahub.org/sites/default/files/resource/unaids-2020-global-aids-factsheets.pdf
UNAIDS. (2022b). HIV estimates with uncertainty bounds 1990-Present [dataset]. Available online: https://www.unaids.org/en/resources/documents/2022/HIV_estimates_with_uncertainty_bounds_1990-present
Solstad S. The pandemic’s true death toll. Economist 2021;20. https://www.economist.com/graphic-detail/coronavirus-excess-deaths-tracker (10 October 2022, date last accessed).
Hasell, J., Mathieu, E., Beltekian, D., Macdonald, B., Giattino, C., Ortiz-Ospina, E., ... & Ritchie, H. (2020). A cross-country database of COVID-19 testing. Scientific data, 7(1), 345. https://www.nature.com/articles/s41597-020-00688-8
Msemburi, W., Karlinsky, A., Knutson, V., Aleshin-Guendel, S., Chatterji, S., & Wakefield, J. (2023). The WHO estimates of excess mortality associated with the COVID-19 pandemic. Nature, 613(7942), 130–137. https://doi.org/10.1038/s41586-022-05522-2
Wang, H., Paulson, K. R., Pease, S. A., Watson, S., Comfort, H., Zheng, P., Aravkin, A. Y., Bisignano, C., Barber, R. M., Alam, T., Fuller, J. E., May, E. A., Jones, D. P., Frisch, M. E., Abbafati, C., Adolph, C., Allorant, A., Amlag, J. O., Bang-Jensen, B., … Murray, C. J. L. (2022). Estimating excess mortality due to the COVID-19 pandemic: A systematic analysis of COVID-19-related mortality, 2020–21. The Lancet, 399(10334), 1513–1536. https://doi.org/10.1016/S0140-6736(21)02796-3
A comparison of estimates can also be found on the website of the Office for National Statistics (ONS) of the UK.
Office for National Statistics (ONS) and Government Office for Science (GOScience). (2022). Comparing Different International Methods of Measuring Excess Mortality. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/articles/comparingdifferentinternationalmeasuresofexcessmortality/2022-12-20
Cite this work
Our articles and data visualizations rely on work from many different people and organizations. When citing this article, please also cite the underlying data sources. This article can be cited as:
Saloni Dattani (2023) - “What were the death tolls from pandemics in history?” Published online at OurWorldinData.org. Retrieved from: 'https://archive.ourworldindata.org/20260222-063515/historical-pandemics.html' [Online Resource] (archived on February 22, 2026).BibTeX citation
@article{owid-historical-pandemics,
author = {Saloni Dattani},
title = {What were the death tolls from pandemics in history?},
journal = {Our World in Data},
year = {2023},
note = {https://archive.ourworldindata.org/20260222-063515/historical-pandemics.html}
}Reuse this work freely
All visualizations, data, and code produced by Our World in Data are completely open access under the Creative Commons BY license. You have the permission to use, distribute, and reproduce these in any medium, provided the source and authors are credited.
The data produced by third parties and made available by Our World in Data is subject to the license terms from the original third-party authors. We will always indicate the original source of the data in our documentation, so you should always check the license of any such third-party data before use and redistribution.
All of our charts can be embedded in any site.