New polio vaccines are key to preventing outbreaks and achieving eradication
To reach the goal of polio eradication, we can use new vaccines to contain outbreaks and improve testing, outbreak responses, and sanitation.
We’ve come a long way in the fight against polio — the infectious disease that used to paralyze hundreds of thousands of people each year. Most of them were children. Eradication is possible, but the last stretch has proven difficult.
Two of the three serotypes (distinct types within a species of virus) of wild poliovirus have already been eradicated.
However, two big challenges remain in crossing the finish line. One is eliminating the last serotype of wild poliovirus. Another is containing vaccine-derived polioviruses, which arose from oral polio vaccines in rare circumstances and spread in some regions where protection against the disease declined.
The world can overcome these hurdles. We can use new vaccines to contain them and improve testing, outbreak responses, and sanitation.
Vaccines have helped us reduce polio cases substantially over time
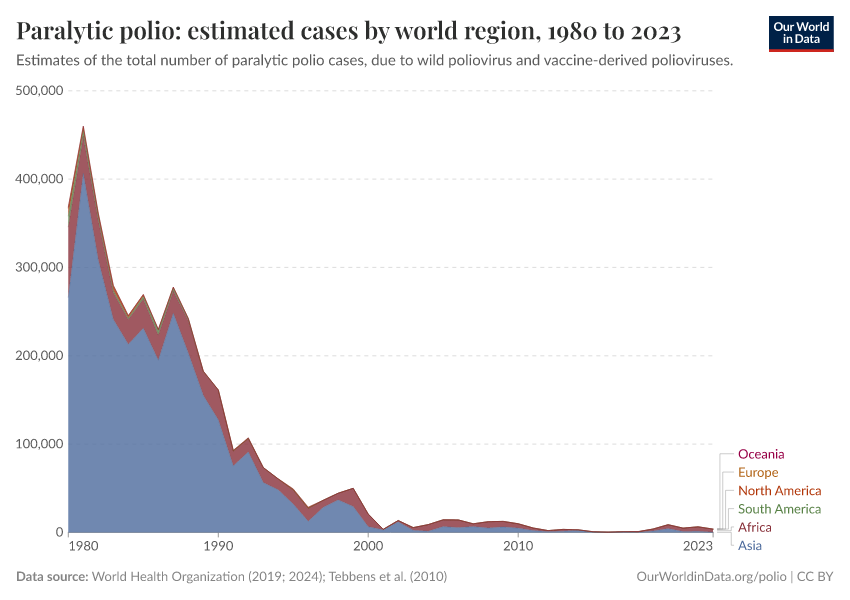
The chart below shows the dramatic decline in polio cases.
This was possible due to effective vaccination efforts with two types of vaccine: inactivated polio vaccines (IPV), developed by Jonas Salk in 1955, and oral polio vaccines (OPV), developed by Albert Sabin in 1961.
Improvements in providing clean water and sanitation have also helped to reduce the spread of poliovirus through contaminated water and food and the risks of other infections, which prevent children from developing immunity against polio.1
In the early 1980s, there were around 400,000 estimated cases annually. In the last few years, there have been around 4,000. That’s a hundred-fold decline. Millions of children have been spared lifelong paralysis.
We are very close to eradicating all three types of wild poliovirus
Wild poliovirus has three serotypes. The three serotypes are distinct types of poliovirus with protein structures that differ sufficiently that protection from one doesn’t protect from the other.
The world has eradicated wild poliovirus serotypes 2 and 3.2
- The last case of wild poliovirus serotype 2 was in 1999 in India, and the WHO declared it eradicated in 2015.
- The last case of wild poliovirus serotype 3 was seen in 2012 in Nigeria, and it was declared eradicated in 2019.3
The world is, therefore, very close to eradicating all serotypes of wild poliovirus globally.
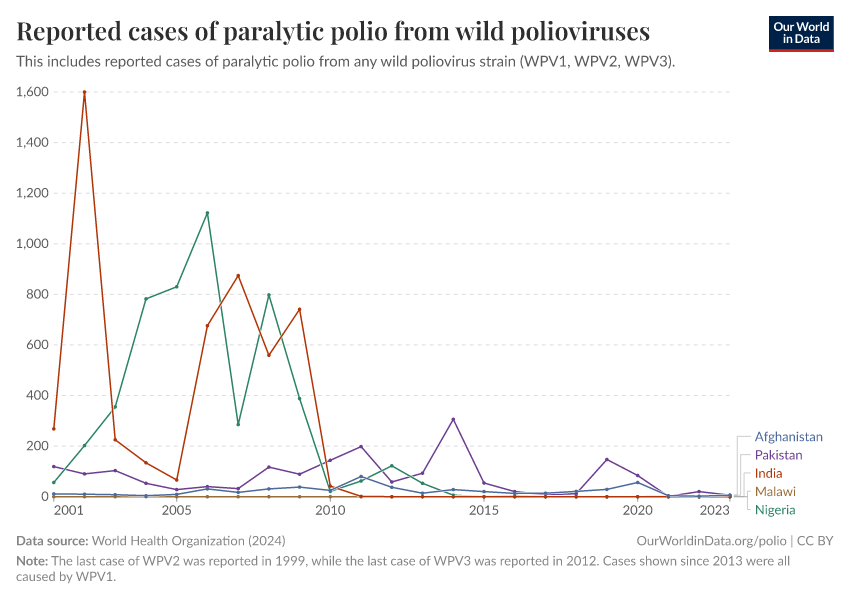
As shown in the map below, only two countries — Afghanistan and Pakistan — are still endemic for wild poliovirus serotype 1 (WPV1).
But as the chart shows, the number of cases is now very low. In 2023, only six cases of wild polio were reported in Afghanistan and another six in Pakistan.
By testing widely to identify potential cases, working with local communities in hard-to-reach areas and at borders, and improving vaccination rates and sanitation, this goal is within reach.4

Most current cases come from vaccine-derived polioviruses
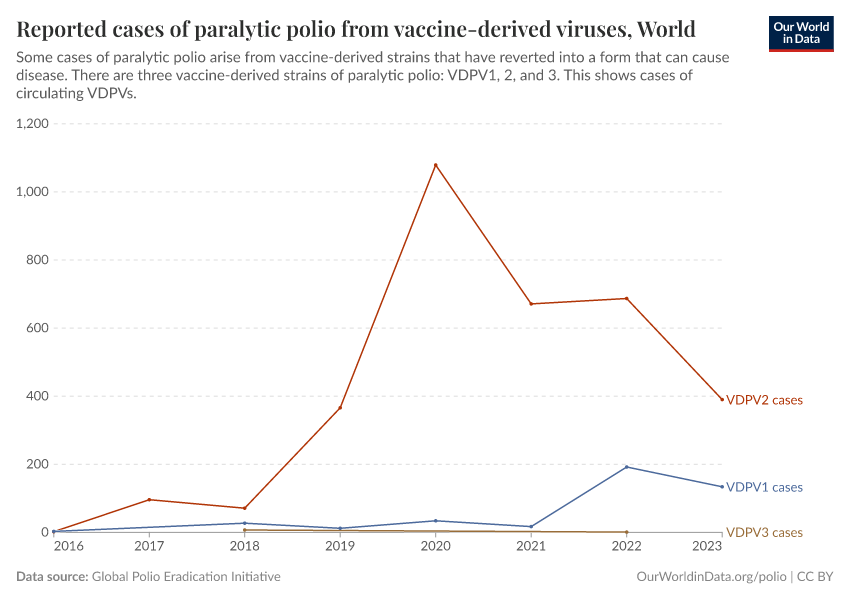
Although the absolute number of cases is much lower than in the past, most cases recently have come from vaccine-derived polioviruses (VDPVs), as shown in the chart below.5
Vaccine-derived polioviruses can arise from the weakened virus in the oral polio vaccine if it has mutated significantly over time in vaccinated people and reverted to the original strain of polio.
Oral polio vaccines are used in poorer countries because they are much easier to administer (as oral drops) and cheaper to manufacture than inactivated polio vaccines, which are given by injection.6
People with immune deficiencies are at higher risk of the vaccine reverting because they can sustain longer infections, giving the virus more time to evolve.7
It can then spread and cause new outbreaks if immunity has fallen in communities. So, somewhat counterintuitively, communities with lower vaccination coverage are more vulnerable to vaccine-derived polio.8

So far, most cases of vaccine-derived poliovirus have come from vaccine-derived polioviruses of serotype 2 — known as VDPV2 — which can mutate faster than other serotypes, making it more likely to revert than other serotypes.9
In addition, there were interruptions in vaccination against polio serotype 2 in 2016, when vaccination against that particular serotype was switched from the oral to the inactivated polio vaccine. It was harder to provide inactivated polio vaccines at scale in poorer regions and reach every child, leading to a rise in cases.10
New vaccines can help prevent further outbreaks
Since 2021, new oral polio vaccines against serotype 2 have been used to prevent further outbreaks of VDPV2.
These are much more genetically stable than the previous oral polio vaccine and much less likely to mutate or potentially revert to the original strain.11
They have already been rolled out widely and helped effectively control outbreaks of VDPV2.12
New oral polio vaccines against serotypes 1 and 3 are still in development.13
In addition, new types of inactivated polio vaccines are also being developed. For example, some candidate vaccines can be administered through skin patches instead of injections. These could be cheaper, easier to provide, and unable to revert to the original strain.14
These new technologies will be crucial in preventing further outbreaks as we approach the ultimate goal of polio eradication.
Let’s close the chapter on polio
To achieve polio eradication, it’s crucial to contain every last case quickly to prevent the spread of polio and protect children from this debilitating disease.
We can use new vaccines, increase polio testing, and improve access to clean water and sanitation.
Together, we can successfully close the chapter on polio, which would be a major victory for humanity.
Acknowledgments
Edouard Mathieu, Max Roser, and Hannah Ritchie provided helpful feedback on this article.
Endnotes
When children have multiple infections in their gut simultaneously, this reduces their ability to develop immunity against polio. This reduces the efficacy of oral polio vaccines and is one reason vaccine efficacy against gut infections tends to be lower in poorer countries.
Parker, E. P. K., Kampmann, B., Kang, G., & Grassly, N. C. (2014). Influence of Enteric Infections on Response to Oral Poliovirus Vaccine: A Systematic Review and Meta-analysis. The Journal of Infectious Diseases, 210(6), 853–864. https://doi.org/10.1093/infdis/jiu182
Global Polio Eradication Initiative. (2019, October 24). Two out of three wild poliovirus strains eradicated. News Stories. https://web.archive.org/web/20191024163941/http://polioeradication.org/news-post/two-out-of-three-wild-poliovirus-strains-eradicated/
U.S. National Authority for Containment of Poliovirus (2023). Polio Disease and Poliovirus Containment. Centers for Disease Control and Prevention. Available online.
In Afghanistan and Pakistan, Pashto-speaking communities are disproportionately affected by polio and represent a large majority of cases.
For around a decade, vaccination was restricted in certain areas of Afghanistan, but since 2021, this ban has been lifted.
Bagcchi, S. (2022). Polio vaccination in Afghanistan. The Lancet Microbe, 3(1), e10. https://doi.org/10.1016/S2666-5247(21)00336-0
World Health Organization, & Global Polio Eradication Initiative. (2021). Polio eradication strategy 2022–2026: Delivering on a promise. Available online.
Unfortunately, global data showing the breakdown of cases by serotype is only available from 2000 onwards.
Thompson, K. M., & Kalkowska, D. A. (2021). Potential Future Use, Costs, and Value of Poliovirus Vaccines. Risk Analysis, 41(2), 349–363. https://doi.org/10.1111/risa.13557
Burns, C. C., Diop, O. M., Sutter, R. W., & Kew, O. M. (2014). Vaccine-Derived Polioviruses. Journal of Infectious Diseases, 210(suppl 1), S283–S293. https://doi.org/10.1093/infdis/jiu295
Macklin, G. R., O’Reilly, K. M., Grassly, N. C., Edmunds, W. J., Mach, O., Santhana Gopala Krishnan, R., Voorman, A., Vertefeuille, J. F., Abdelwahab, J., Gumede, N., Goel, A., Sosler, S., Sever, J., Bandyopadhyay, A. S., Pallansch, M. A., Nandy, R., Mkanda, P., Diop, O. M., & Sutter, R. W. (2020). Evolving epidemiology of poliovirus serotype 2 following withdrawal of the serotype 2 oral poliovirus vaccine. Science, 368(6489), 401–405. https://doi.org/10.1126/science.aba1238
Famulare, M., Chang, S., Iber, J., Zhao, K., Adeniji, J. A., Bukbuk, D., Baba, M., Behrend, M., Burns, C. C., & Oberste, M. S. (2016). Sabin Vaccine Reversion in the Field: A Comprehensive Analysis of Sabin-Like Poliovirus Isolates in Nigeria. Journal of Virology, 90(1), 317–331. https://doi.org/10.1128/JVI.01532-15
Researchers attribute vaccine-derived poliovirus outbreaks after 2016 to interruptions in vaccination rates that occurred after the global “switch” in 2016, where countries moved away from using oral polio vaccines for serotype 2 to using inactivated polio vaccines for serotype 2.
Although inactivated polio vaccines don’t carry a risk of reversion because they are more expensive and difficult to administer, this reduced vaccination coverage against serotype 2 after that period, leading to a loss of population immunity and easier spread of VDPV2.
Pons-Salort, M., Burns, C. C., Lyons, H., Blake, I. M., Jafari, H., Oberste, M. S., Kew, O. M., & Grassly, N. C. (2016). Preventing Vaccine-Derived Poliovirus Emergence during the Polio Endgame. PLOS Pathogens, 12(7), e1005728. https://doi.org/10.1371/journal.ppat.1005728
Macklin, G. R., O’Reilly, K. M., Grassly, N. C., Edmunds, W. J., Mach, O., Santhana Gopala Krishnan, R., Voorman, A., Vertefeuille, J. F., Abdelwahab, J., Gumede, N., Goel, A., Sosler, S., Sever, J., Bandyopadhyay, A. S., Pallansch, M. A., Nandy, R., Mkanda, P., Diop, O. M., & Sutter, R. W. (2020). Evolving epidemiology of poliovirus serotype 2 following withdrawal of the serotype 2 oral poliovirus vaccine. Science, 368(6489), 401–405. https://doi.org/10.1126/science.aba1238
Oral and inactivated polio vaccines are believed to have similar efficacy in preventing paralytic polio, although their efficacy varies by region. Oral vaccines are more likely to prevent ‘shedding’ of the virus in the stool, which could be more important in some regions.
Estivariz, C. F., Kovacs, S. D., & Mach, O. (2023). Review of use of inactivated poliovirus vaccine in campaigns to control type 2 circulating vaccine derived poliovirus (cVDPV) outbreaks. Vaccine, 41, A113–A121. https://doi.org/10.1016/j.vaccine.2022.03.027
Grassly, N. C. (2014). Immunogenicity and Effectiveness of Routine Immunization With 1 or 2 Doses of Inactivated Poliovirus Vaccine: Systematic Review and Meta-analysis. The Journal of Infectious Diseases, 210(suppl_1), S439–S446. https://doi.org/10.1093/infdis/jit601
Hird, T. R., & Grassly, N. C. (2012). Systematic Review of Mucosal Immunity Induced by Oral and Inactivated Poliovirus Vaccines against Virus Shedding following Oral Poliovirus Challenge. PLoS Pathogens, 8(4), e1002599. https://doi.org/10.1371/journal.ppat.1002599
Konopka-Anstadt, J. L., Campagnoli, R., Vincent, A., Shaw, J., Wei, L., Wynn, N. T., Smithee, S. E., Bujaki, E., Te Yeh, M., Laassri, M., Zagorodnyaya, T., Weiner, A. J., Chumakov, K., Andino, R., Macadam, A., Kew, O., & Burns, C. C. (2020). Development of a new oral poliovirus vaccine for the eradication end game using codon deoptimization. Npj Vaccines, 5(1), 26. https://doi.org/10.1038/s41541-020-0176-7
Bandyopadhyay, A. S., & Zipursky, S. (2023). A novel tool to eradicate an ancient scourge: The novel oral polio vaccine type 2 story. The Lancet Infectious Diseases, 23(2), e67–e71. https://doi.org/10.1016/S1473-3099(22)00582-5
Ochoge, M., Futa, A. C., Umesi, A., Affleck, L., Kotei, L., Daffeh, B., Saidy-Jah, E., Njie, A., Oyadiran, O., Edem, B., Jallow, M., Jallow, E., Donkor, S. A., Tritama, E., Abid, T., Jones, K. A. V., Mainou, B. A., Konz, J. O., Fix, A., … Clarke, E. (2024). Safety of the novel oral poliovirus vaccine type 2 (nOPV2) in infants and young children aged 1 to <5 years and lot-to-lot consistency of the immune response to nOPV2 in infants in The Gambia: A phase 3, double-blind, randomised controlled trial. The Lancet, 403(10432), 1164–1175. https://doi.org/10.1016/S0140-6736(23)02844-1
Bandyopadhyay, A. S., Cooper, L. V., & Zipursky, S. (2024). One billion doses and WHO prequalification of nOPV2: Implications for the global polio situation and beyond. PLOS Global Public Health, 4(2), e0002920. https://doi.org/10.1371/journal.pgph.0002920
Yeh, M. T., Smith, M., Carlyle, S., Konopka-Anstadt, J. L., Burns, C. C., Konz, J., Andino, R., & Macadam, A. (2023). Genetic stabilization of attenuated oral vaccines against poliovirus types 1 and 3. Nature, 619(7968), 135–142. https://doi.org/10.1038/s41586-023-06212-3
Kumar, P., Bird, C., Holland, D., Joshi, S. B., & Volkin, D. B. (2022). Current and next-generation formulation strategies for inactivated polio vaccines to lower costs, increase coverage, and facilitate polio eradication. Human Vaccines & Immunotherapeutics, 18(7), 2154100. https://doi.org/10.1080/21645515.2022.2154100
Cite this work
Our articles and data visualizations rely on work from many different people and organizations. When citing this article, please also cite the underlying data sources. This article can be cited as:
Saloni Dattani (2024) - “New polio vaccines are key to preventing outbreaks and achieving eradication” Published online at OurWorldinData.org. Retrieved from: 'https://archive.ourworldindata.org/20260119-065148/new-polio-vaccines-are-key-to-preventing-outbreaks-and-achieving-eradication.html' [Online Resource] (archived on January 19, 2026).BibTeX citation
@article{owid-new-polio-vaccines-are-key-to-preventing-outbreaks-and-achieving-eradication,
author = {Saloni Dattani},
title = {New polio vaccines are key to preventing outbreaks and achieving eradication},
journal = {Our World in Data},
year = {2024},
note = {https://archive.ourworldindata.org/20260119-065148/new-polio-vaccines-are-key-to-preventing-outbreaks-and-achieving-eradication.html}
}Reuse this work freely
All visualizations, data, and code produced by Our World in Data are completely open access under the Creative Commons BY license. You have the permission to use, distribute, and reproduce these in any medium, provided the source and authors are credited.
The data produced by third parties and made available by Our World in Data is subject to the license terms from the original third-party authors. We will always indicate the original source of the data in our documentation, so you should always check the license of any such third-party data before use and redistribution.
All of our charts can be embedded in any site.