New drugs approved in the United States
What you should know about this indicator
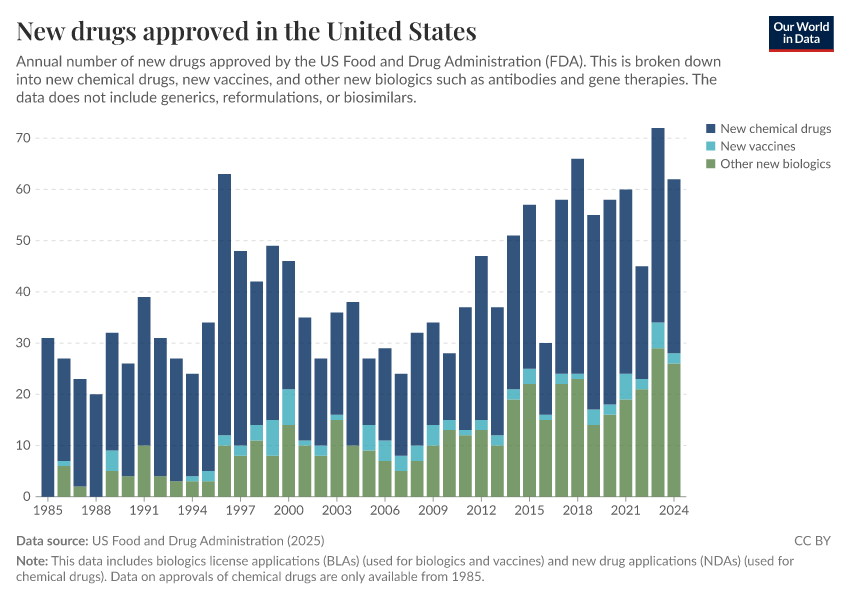
This data includes both new drug applications (NDAs) and biologics license applications (BLAs) approved by the Center for Drug Evaluation and Research (CDER) and the Center for Biologics Evaluation and Research (CBER) of the FDA.
What you should know about this indicator
This data includes both new drug applications (NDAs) and biologics license applications (BLAs) approved by the Center for Drug Evaluation and Research (CDER) and the Center for Biologics Evaluation and Research (CBER) of the FDA.
Sources and processing
This data is based on the following sources
How we process data at Our World in Data
All data and visualizations on Our World in Data rely on data sourced from one or several original data providers. Preparing this original data involves several processing steps. Depending on the data, this can include standardizing country names and world region definitions, converting units, calculating derived indicators such as per capita measures, as well as adding or adapting metadata such as the name or the description given to an indicator.
At the link below you can find a detailed description of the structure of our data pipeline, including links to all the code used to prepare data across Our World in Data.
Notes on our processing step for this indicator
- Biologicals approved by the CBER have been extracted from the FDA's "Purple Book", which lists all licensed biological products.
- CDER approvals (which include some biologics and all chemical drugs) come directly from the "Compilation of CDER NME and New Biologic Approvals" database, which is published by CDER.
- This dataset only contains new drugs (new chemical entities and biologics). We removed biosimilars and generics from the dataset, by excluding the "biosimilar" or "interchangeable" license types as well as products with a reference product listed.
Reuse this work
- All data produced by third-party providers and made available by Our World in Data are subject to the license terms from the original providers. Our work would not be possible without the data providers we rely on, so we ask you to always cite them appropriately (see below). This is crucial to allow data providers to continue doing their work, enhancing, maintaining and updating valuable data.
- All data, visualizations, and code produced by Our World in Data are completely open access under the Creative Commons BY license. You have the permission to use, distribute, and reproduce these in any medium, provided the source and authors are credited.
Citations
How to cite this page
To cite this page overall, including any descriptions, FAQs or explanations of the data authored by Our World in Data, please use the following citation:
“Data Page: New drugs approved in the United States”, part of the following publication: Tuna Acisu, Saloni Dattani, Fiona Spooner, Veronika Samborska, Hannah Ritchie, and Max Roser (2025) - “Medicine and Biotechnology”. Data adapted from US Food and Drug Administration. Retrieved from https://archive.ourworldindata.org/20260304-094028/grapher/new-drugs-approved-in-the-united-states.html [online resource] (archived on March 4, 2026).How to cite this data
In-line citationIf you have limited space (e.g. in data visualizations), you can use this abbreviated in-line citation:
US Food and Drug Administration (2025) – with major processing by Our World in DataFull citation
US Food and Drug Administration (2025) – with major processing by Our World in Data. “New drugs approved in the United States” [dataset]. US Food and Drug Administration, “CDER New Molecular Entity (NME) Drug and New Biologic Approvals” [original data]. Retrieved March 7, 2026 from https://archive.ourworldindata.org/20260304-094028/grapher/new-drugs-approved-in-the-united-states.html (archived on March 4, 2026).