Average clinical trial study length, by phase
What you should know about this indicator
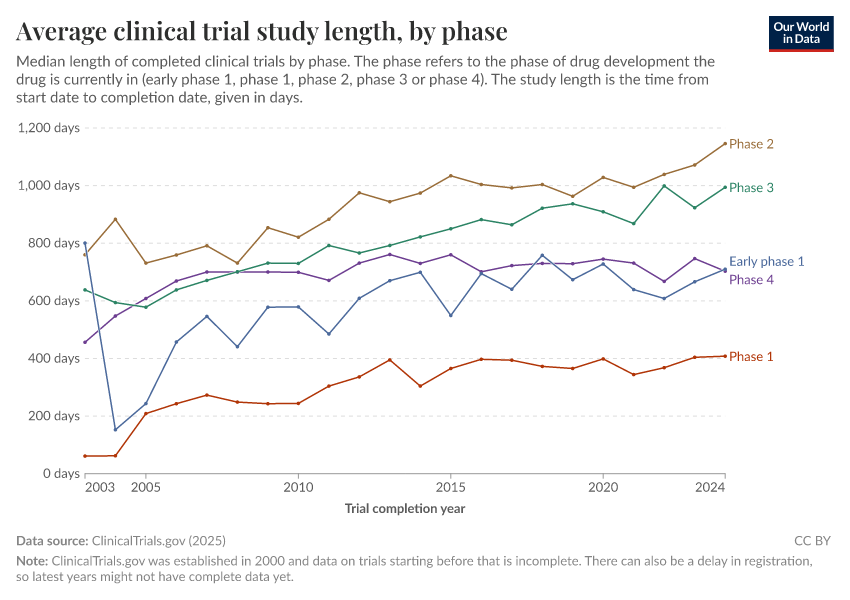
- Clinical trials are conducted in different phases of the development of a new treatment or intervention, such as early phase 1, phase 1, phase 2, phase 3, or phase 4.
- Early phase 1 trials are exploratory trials that test the effect of an intervention on the human body, often with a small number of participants. Not every treatment has an early phase 1 trial.
- Phase 1 trials test the safety and dosage of a new treatment, phase 2 trials test the effectiveness and side effects, and phase 3 trials compare the new treatment to standard treatments.
- Phase 4 trials are conducted after a treatment has been approved and are used to monitor the long-term effects and side-effects of the treatment. Not every treatment has a phase 4 trial.
- "Phase 1/phase 2" trials are a combination of phase 1 and phase 2 trials, and "phase 2/phase 3" trials are a combination of phase 2 and phase 3 trials.
- The average length is calculated by taking the difference between the start date and completion date of each trial, grouped by phase and completion year.
- This data comes from the ClinicalTrials.gov database. It only includes interventional clinical trials that are marked as "completed" and have a valid "completion date".
- Registration in the ClinicalTrials.gov database is mandatory for trials in the United States and for treatments that seek FDA approval, but voluntary for other trials conducted in other countries.
What you should know about this indicator
- Clinical trials are conducted in different phases of the development of a new treatment or intervention, such as early phase 1, phase 1, phase 2, phase 3, or phase 4.
- Early phase 1 trials are exploratory trials that test the effect of an intervention on the human body, often with a small number of participants. Not every treatment has an early phase 1 trial.
- Phase 1 trials test the safety and dosage of a new treatment, phase 2 trials test the effectiveness and side effects, and phase 3 trials compare the new treatment to standard treatments.
- Phase 4 trials are conducted after a treatment has been approved and are used to monitor the long-term effects and side-effects of the treatment. Not every treatment has a phase 4 trial.
- "Phase 1/phase 2" trials are a combination of phase 1 and phase 2 trials, and "phase 2/phase 3" trials are a combination of phase 2 and phase 3 trials.
- The average length is calculated by taking the difference between the start date and completion date of each trial, grouped by phase and completion year.
- This data comes from the ClinicalTrials.gov database. It only includes interventional clinical trials that are marked as "completed" and have a valid "completion date".
- Registration in the ClinicalTrials.gov database is mandatory for trials in the United States and for treatments that seek FDA approval, but voluntary for other trials conducted in other countries.
Sources and processing
This data is based on the following sources
How we process data at Our World in Data
All data and visualizations on Our World in Data rely on data sourced from one or several original data providers. Preparing this original data involves several processing steps. Depending on the data, this can include standardizing country names and world region definitions, converting units, calculating derived indicators such as per capita measures, as well as adding or adapting metadata such as the name or the description given to an indicator.
At the link below you can find a detailed description of the structure of our data pipeline, including links to all the code used to prepare data across Our World in Data.
Notes on our processing step for this indicator
- First we calculate the length of each trial by taking the difference between the start date and completion date of each trial.
- Then we group the trials by phase and completion year, and calculate the median length of each group.
Reuse this work
- All data produced by third-party providers and made available by Our World in Data are subject to the license terms from the original providers. Our work would not be possible without the data providers we rely on, so we ask you to always cite them appropriately (see below). This is crucial to allow data providers to continue doing their work, enhancing, maintaining and updating valuable data.
- All data, visualizations, and code produced by Our World in Data are completely open access under the Creative Commons BY license. You have the permission to use, distribute, and reproduce these in any medium, provided the source and authors are credited.
Citations
How to cite this page
To cite this page overall, including any descriptions, FAQs or explanations of the data authored by Our World in Data, please use the following citation:
“Data Page: Average clinical trial study length, by phase”, part of the following publication: Tuna Acisu, Saloni Dattani, Fiona Spooner, Veronika Samborska, Hannah Ritchie, and Max Roser (2025) - “Medicine and Biotechnology”. Data adapted from ClinicalTrials.gov. Retrieved from https://archive.ourworldindata.org/20260225-182047/grapher/average-study-length-by-phase.html [online resource] (archived on February 25, 2026).How to cite this data
In-line citationIf you have limited space (e.g. in data visualizations), you can use this abbreviated in-line citation:
ClinicalTrials.gov (2025) – with major processing by Our World in DataFull citation
ClinicalTrials.gov (2025) – with major processing by Our World in Data. “Average clinical trial study length, by phase” [dataset]. ClinicalTrials.gov, “Clinical Trials (ClinicalTrials.gov)” [original data]. Retrieved March 3, 2026 from https://archive.ourworldindata.org/20260225-182047/grapher/average-study-length-by-phase.html (archived on February 25, 2026).